Sherlock Biosciences announces initial results of automation study with NorDx Laboratories on CRISPR-based test for SARS-CoV-2
Leading clinical laboratory runs first-ever successful automation of a CRISPR test for high-throughput, using Sherlock’s SARS-CoV-2 process
26 Jul 2021
Sherlock Biosciences, an Engineering Biology company dedicated to making diagnostic testing better, faster and more affordable, has announced initial results from an automation study of its CRISPR-based test for SARS-CoV-2 in partnership with NorDx Laboratories, a member of MaineHealth – a recognized leader in New England and Maine’s largest integrated healthcare system. The study is the first-ever successful automation of a CRISPR test for high-throughput, and automating Sherlock’s assay enables laboratories to test clinical samples from thousands of patients a day on a single system, streamlining workflows and improving patient outcomes.
“We believe that Sherlock’s CRISPR-based technology holds promise as a potential answer to high volume testing needs,” said Robert Carlson, M.D., medical director at NorDx. “Fighting the pandemic requires testing methods that can be easily automated for high-throughput and rapidly adapt to new and emerging variants.”
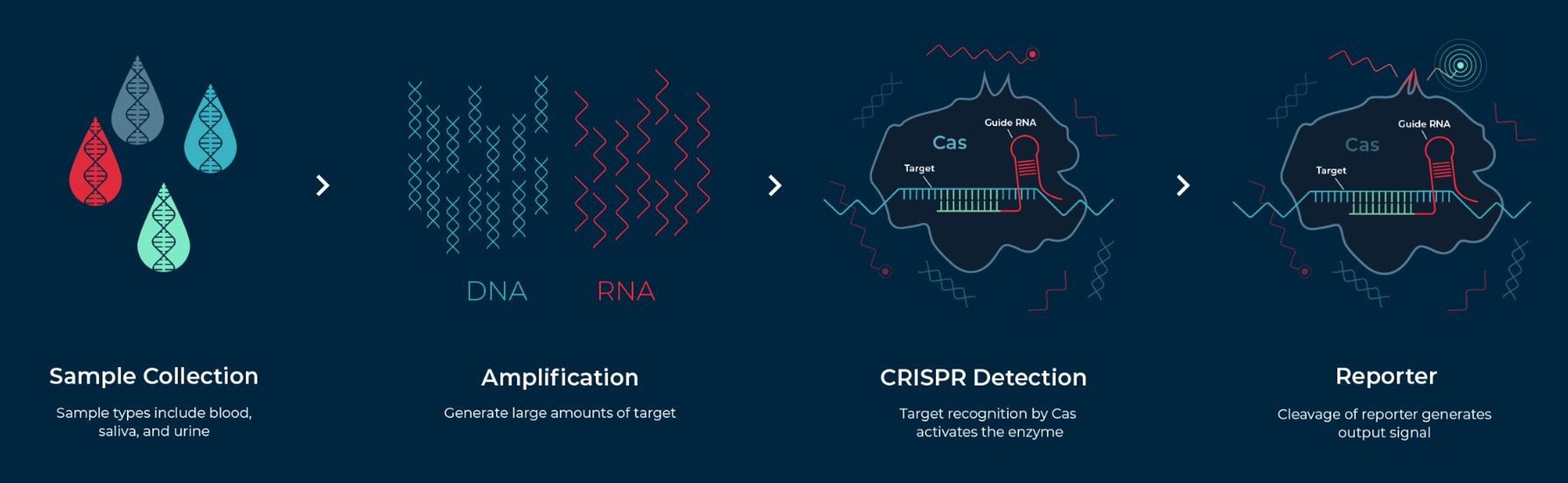
The Sherlock CRISPR SARS-CoV-2 test kit is designed for use in laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests. Based on the SHERLOCK method, which stands for Specific High-sensitivity Enzymatic Reporter unLOCKing, the kit works by programming a CRISPR nuclease to detect the presence of a specific genetic signature – in this case, the genetic signature for SARS-CoV-2 – in a nasal swab, nasopharyngeal swab, oropharyngeal swab or bronchoalveolar lavage (BAL) specimen. When the signature is found, the CRISPR enzyme is activated and cuts reporter RNAs provided as part of the kit to release a detectable signal, yielding results in about an hour.
“Because Sherlock holds the most comprehensive IP position in CRISPR diagnostics, we are in a unique position to enable partners to achieve highly-automated and point-of-care solutions for any molecular diagnostic test” said Rahul Dhanda, co-founder, president and CEO of Sherlock Biosciences. “We are excited by these initial results and look forward to partnering with the NorDx team to further demonstrate that our robust method can be easily automated to provide a fast, user-friendly and inexpensive method for containing the pandemic through rapid and accurate detection of SARS-CoV-2.”
Do you use Sherlock Biosciences products in your lab? Write a review today for your chance to win a $400 Amazon gift card>>