Streamlined Clinical Mass Spectrometry Workflow at Imperial College Healthcare
An optimized, automated workflow enables continued growth of the clinical mass spectrometry service at this leading London Trust
20 Feb 2018

Emma Walker is a Consultant Clinical Scientist, and Specialty Lead for Diagnostic Endocrinology at North West London Pathology, hosted by Imperial College Healthcare NHS Trust. Her laboratory has recently implemented a fully automated liquid chromatography tandem mass spectrometry (LC-MS/MS) workflow. SelectScience speaks to Emma to learn more about the service.
SS: Could you tell us about your job responsibilities and place of work?
EW: I work for Imperial College Healthcare in the north west of London, UK. This is a very large trust, incorporating five hospitals and serving a population of 2 million. Last year, according to our latest annual report, we dealt with 1.5 million patient episodes and pathology processed 15 million specimens.
My role is to oversee the diagnostic endocrine service which includes the clinical assays that we carry out using LC-MS/MS, such as steroid and peptide hormone analysis. Initially we were using radioimmunoassays (RIAs), however as a longstanding project, we’ve been taking the in-house immunoassays and moving them over to LC-MS/MS technology. As our workload has increased, we have had to adapt and think very carefully about our workflow through the lab. Our throughput is now much higher than it used to be and our service has had to evolve accordingly. My role as the service lead involves me determining the future vision and strategic direction of the department, implementing new technologies, and ensuring a smooth transmission.
SS: Can you describe your LC-MS/MS service, when was it implemented and what advantages does it offer over traditional immunoassay testing?
EW: Our lab first acquired the Waters mass spectrometry (MS) instruments a decade ago, when there was an explosion of vitamin D testing. The only other method for vitamin D testing at the time was RIA, but the kits were labor intensive and too slow to allow us to meet our desired turnaround times. This fact enabled us to justify the purchase of our MS instrumentation. When we first moved to LC-MS/MS the process was still fairly labor intensive and very manual, although we benefited from increased sensitivity, specificity and accuracy. We had two LC-MS/MS analyzers, but the manual extraction and processing procedures were a real bottleneck for analysis.
In 2010, when immunoassay vitamin D kits started to be produced, we went to a two-strand approach. We carried out immunoassay testing on the samples first, and then we used the LC-MS/MS for confirmation testing of high values, and for the monitoring of people taking vitamin D supplements. The demand for vitamin D testing continued to go up and up, and we also wanted to free up capacity to develop and carry out more specialist testing using our systems. It became increasingly clear that in order to do this, we needed to streamline our workflow.
Optimizing the workflow
Initially we developed a new MS method for female testosterone in 2012, which we wanted to automate and integrate into our workflow. But at that time we didn’t have the funding or justification for a business case. Then in 2014, the manufacturer of the RIA kit we were using for aldosterone discontinued the product line, so we could no longer use it. At that point, we put together a business plan to implement a Waters LC-MS/MS workflow coupled to an automated sample preparation system. In 2015 we had installed the equipment needed to automate our LC-MS/MS workflow.
We now have two Waters ACQUITY® TQDs, which we use routinely for vitamin D and steroid analysis. We also have a Waters ACQUITY UPLC® I-Class / Xevo® TQ-S IVD System – on which we currently run our aldosterone assay and also an angiotensin 1 assay for the measurement of plasma renin activity. We will also shortly be adding plasma metanephrines to our test repertoire. All of our instruments are at full capacity and running more or less continuously.

Seamless LIMS integration
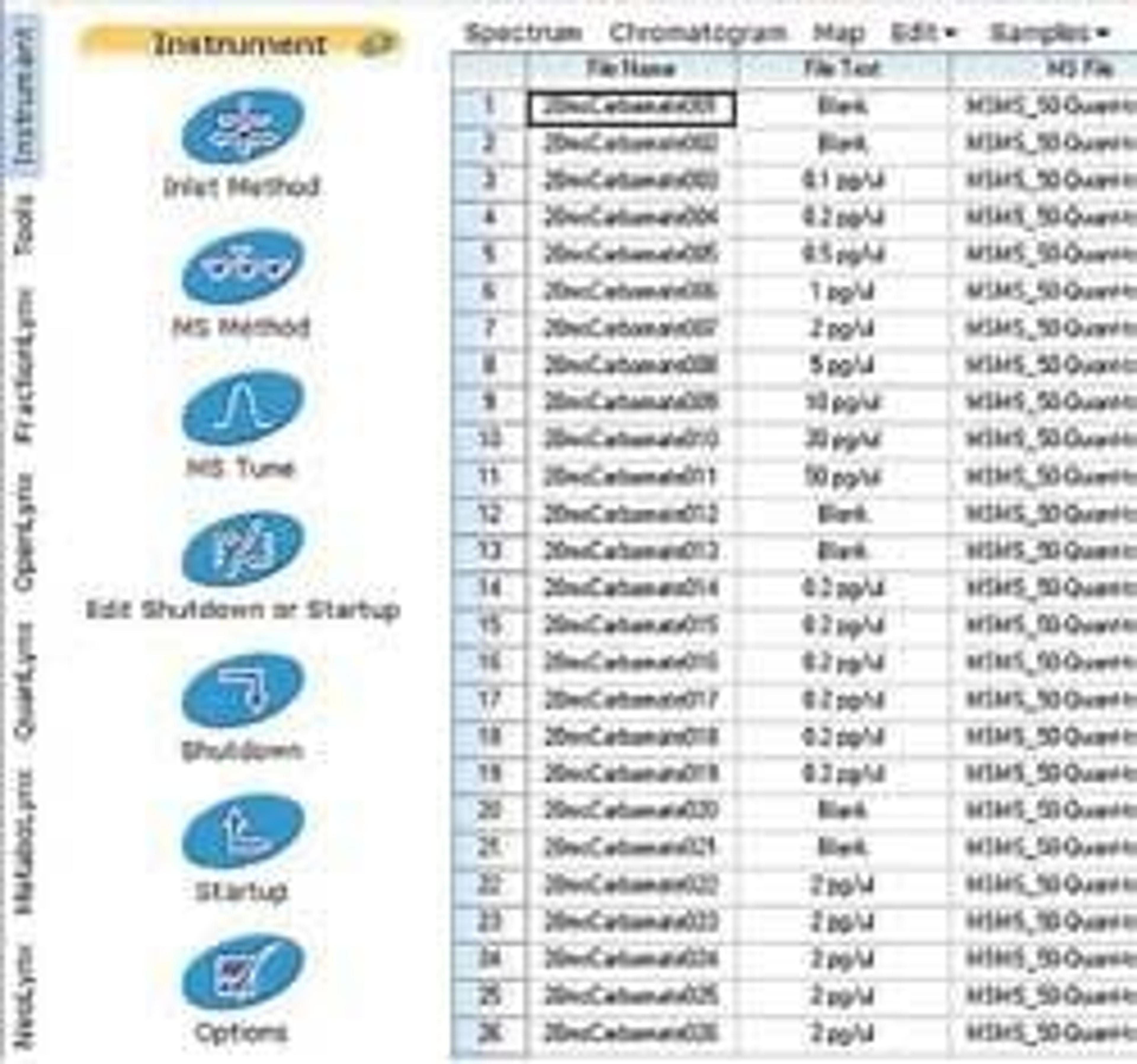
Another important part of our workflow is the integration of our MS instruments into the wider IT structure of the lab, with full interfacing between our LIMS and the analyzers. Prior to this we were manually inputting data, but with this fully integrated approach, the data is automatically processed and exported to the LIMS. The MassLynx® Mass Spectrometry Software, coupled with automated front-end and analytical extraction, makes our whole workflow highly streamlined.
The optimization of our workflow has freed up the time of qualified personnel for new method development, and for the continued growth of the service. In fact, we have been so successful at this that we are hoping to go back out to tender in the near future to acquire another MS instrument.
SS: How was the implementation process, what advice can you offer to other labs looking to move over to LC-MS/MS?
EW: My advice is to begin with the end in mind. Acquire every aspect of the system upfront. Think about what your integrated workflow will look like, and work with your chosen manufacturer to ensure that the workflow will be fit for purpose. It is much harder to build as you grow, so I recommend that labs take an automated, integrated approach from day one. Think beyond the instrument that you want to buy, and consider what your service really needs. We had fantastic support from Waters, to ensure that we were fully equipped and supported from the very beginning. We also had support from the Waters clinical team, who really understood the needs of the clinical lab.
Find out more about Waters family of LC-MS/MS in vitro diagnostic medical devices