The complement system provides promise of novel prognostic biomarkers for cancer
The context-dependent role of the complement system in cancer has the potential to provide novel prognostic biomarkers to inform treatment decisions for cancer patients
28 Sept 2023
Prof. Dr. Lubka Roumenina discusses the complex role of the complement system in cancer
Investigating the role of the complement system in cancer biology could lead to the discovery of novel prognostic biomarkers.
The complement system is a crucial component of the immune system comprising proteins that aid in the defense against infections and promoting the removal of damaged cells.
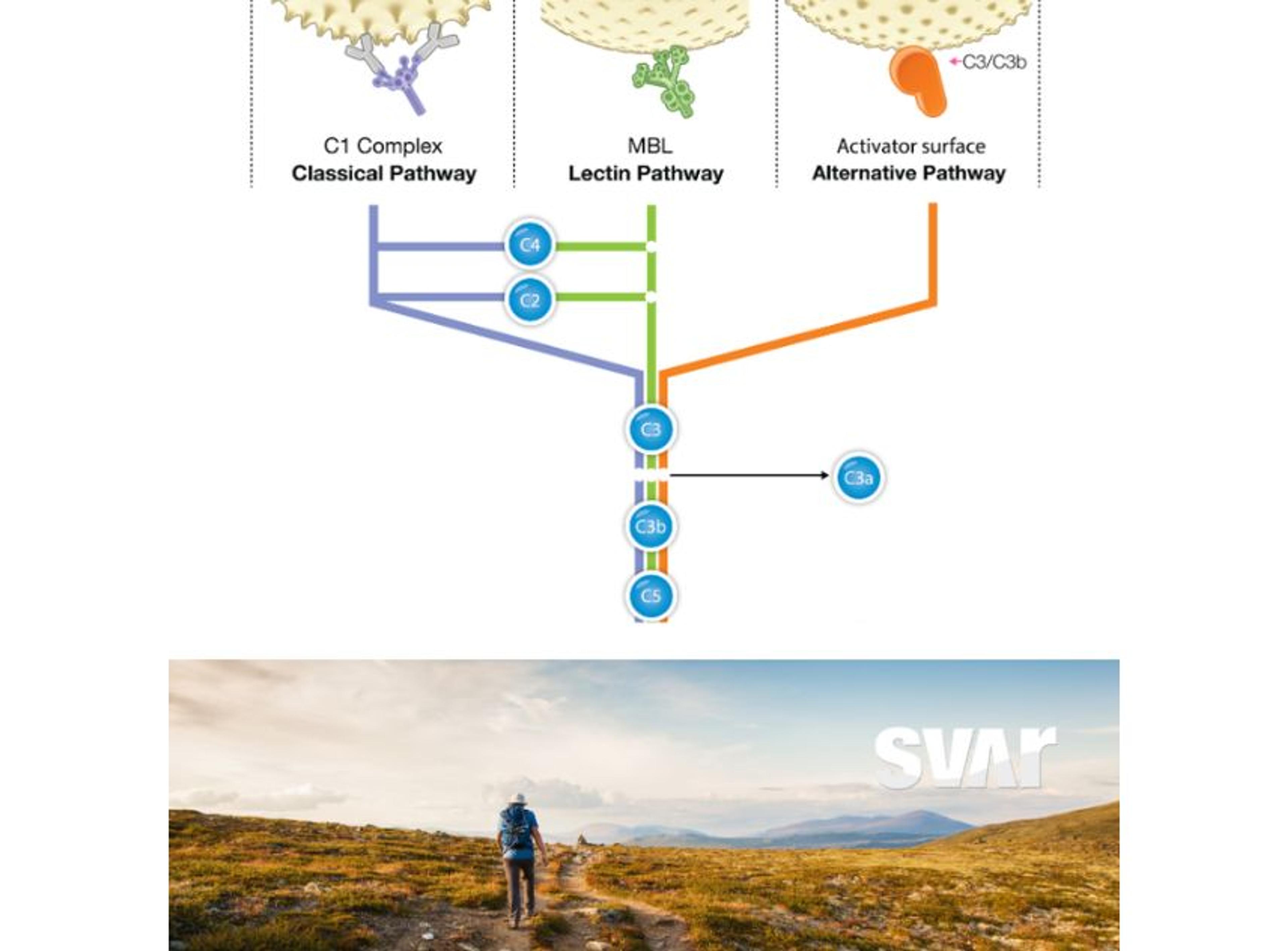
This defense system plays a significant role in cancer. While complement activation can lead to tumor cell killing, chronic inflammation induced by complement and complement peptides (anaphylatoxins) can be pro-tumoral, fostering cancer growth1. Recent research has also revealed non-canonical properties of complement proteins, such as neoangiogenesis and intracellular regulation of the cell metabolism. This can also have consequences for cancer progression.
This complex and conflicting role of complement in the tumor microenvironment presents challenges to fully harness its therapeutic potential for cancer patients. It has become evident that the role of complement in cancer is highly dependent on the specific cancer type and therapeutic strategy. Understanding complement's multiple states and functions is crucial in comprehending its complex role in cancer progression and clinical outcome.
Read on to hear Prof. Dr. Lubka Roumenina, Senior Scientist at Inserm, the French National Institute of Health and Medical Research, and Deputy Director of the Inflammation, Complement, and Cancer Team in the Cordeliers Research Center, Paris, discuss this context-dependent role of complement in cancer and how this can be harnessed to help treat and manage cancer patients. Plus, she provides insights into the importance of immunoassays in this research.
Prognostic biomarkers can predict treatment outcomes

Prof. Dr. Lubka Roumenina and her team working in the lab
“Complement's role in cancer has long been overlooked due to initial contradictory findings,” explains Roumenina. However, the complement system is now recognized to have implications in many of the hallmarks of cancer. Roumenina continues, “the context-dependence of complement adds complexity, but is also exciting as it allows targeted therapeutic approaches specific to certain cell types, without impacting others such as healthy cells.”
This work is important to learn how complement biomarkers can be harnessed in the context of cancer. For example, C4d is a biomarker that reflects the terminal cleavage state of C4, an activation product of the classical and lectin pathway. “Remarkably, we found that this C4d biomarker exhibits a strong link with poor prognosis among renal cancer patients when assessed at the time of surgery2,” Roumenina explains. In the first three years of follow-up, the majority of patients with normal levels showed no cancer progression, while a significant proportion of those with elevated levels did progress. This biomarker has also been documented to correlate with adverse prognosis in lung cancer and malignant mesothelioma.
“There's potential for C4d to become a routine component in complement exploration, particularly for cancer cases,” says Roumenina. “However, this assertion is based on initial outcomes from small cohorts.” Its utility as a predictor of poor prognosis and its capability to forecast responses to treatments like immunotherapy or chemotherapy need validation in larger, real-world cohorts across diverse cancer types. While further investigation is necessary before establishing its relevance as a diagnostic standard, the initial findings show great promise.
The terminal complement complex (TCC) is another biomarker of interest as one may expect elevated levels in cancer patients due to complement activation. However, surprisingly, elevated levels are not commonly observed in most cancer patients. Roumenina says, “this phenomenon mirrors the tumor's immune evasion strategy, which prevents the complete progression of the complement cascade.” She adds, “nonetheless, under specific treatment regimens like monoclonal antibodies, heightened TCC could serve as a potential biomarker. It could signify successful cascade completion, leading to effective tumor cell destruction. This must be further investigated.”
Reliable biomarkers fuel ongoing research
Ongoing research seeks to characterize the context-dependent role of complement in different cancer types. This aims to identify novel prognostic biomarkers, enabling personalized treatment approaches. Immunoassays play a crucial role in this research, aiding in a better understanding of complement in the tumor microenvironment and facilitating significant advancements in the field.
Roumenina explains, “to investigate complement's roles in both physiology and pathology, we must investigate its dynamics in human systems, as mice and in vitro models offer limited insight into in vivo human scenarios.” This requires reliable biomarkers to measure complement's activity across diverse pathological contexts. Roumenina and her team are developing an integrated ‘complementomics’ approach, adapting tools to measure complement across all compartments, activation states, and components, including complement proteins, activation products, anti-complement proteins, autoantibodies, and complement receptors. The aim is to construct a comprehensive understanding of how complement drives physiological and pathological processes.
Reliable biomarkers are essential to precision medicine.
Prof. Dr. Lubka Roumenina Inserm and The Cordeliers Research Center
The significance of complement proteins in cancer is beginning to become more recognized and there are several ongoing clinical trials exploring complement-based therapeutics, administered to cancer patients singly or in conjunction with checkpoint inhibitors. These trials hold immense promise, offering crucial insights into the potential of complement as a cancer therapeutic.
“Patient selection is of utmost importance, as complement inhibitors might not be effective for individuals lacking local complement activation,” Roumenina states. “Thus, reliable biomarkers are essential to precision medicine, aiding in identifying the most suitable complement therapeutic for each patient, given the diverse nature of tumors.”
The recognition of tumor heterogeneity as a driver for therapeutic evasion underscores the need for dependable biomarkers. This could be used to stratify patients who could benefit from complement-based interventions, be they activators or inhibitors, within specific contexts.
There is a growing realization of the significant role the complement system plays in a multitude of diseases, and continued investigation holds the potential to uncover its precise mechanisms in disease pathogenesis and its value as a target for therapeutic interventions.
Svar Complement Biomarker Assays offer crucial insights to the roles complement plays in diseases such as cancer. These assays specifically target neoepitopes unique to activated complement components or complexes to enable a quick and reliable determination of complement proteins such as TCC and C4d.
The WIESLAB® Functional Complement Assays are another tool from Svar Life Science that accurately characterize the three pathways of the complement system through fast ELISA-based analysis. These offer pathway-specific insights without cross-pathway interference, making them essential for drug development and clinical diagnostics.
Find out more about the assay solutions and services offered by Svar Life Science>>
References:
Roumenina L.T., Daugan M.V., Petitprez F., Sautès-Fridman C., Fridman W.H. Context-dependent roles of complement in cancer. Nat Rev Cancer 698-715 (2019). https://doi,org/10.1038/s41568-019-0210-0 Daugan M.V., Revel M., Russick J., Dragon-Durey M.A., Gaboriaud C., Robe-Rybkine T., Poillerat V., Grunenwald A., Lacroix G., Bougouin A., Meylan M., Verkarre V., Oudard S.M., Mejean A., Vano Y.A., Perkins G., Validire P., Cathelineau X., Sanchez-Salas R., Damotte D., Fremeaux-Bacchi V., Cremer I., Sautès-Fridman C., Fridman W.H., Roumenina L.T. Complement C1s and C4d as Prognostic Biomarkers in Renal Cancer: Emergence of Noncanonical Functions of C1s. Immunol Res. 891-90 (2021). https://doi.org/10.1158/2326-6066.CIR-20-0532