The International Myeloma Working Group Guidelines for the Diagnosis of Multiple Myeloma
A reminder of the most important updates to the Multiple Myeloma guidelines
17 Mar 2016

Dr. Dispenzieri, Professor of Medicine in the Division of Hematology at the Mayo Clinic, discusses the International Myeloma Working Group guidelines for the diagnosis of myeloma, and the new biomarker status of the Freelite assay.
The International Myeloma Working Group (IMWG) issued updated guidelines at the end of 2014 for the diagnosis of Multiple Myeloma (MM). One of the most important updates to the guidelines is the incorporation of ‘high-risk’ Monoclonal Gammopathy of Undetermined Significance (MGUS) patients into the ‘active Myeloma’ category.
New biomarker inclusions
In addition, the guidelines were also updated to include three new biomarkers that can be used as ‘Myeloma Defining Events’ (MDEs), in addition to the classic “CRAB” features (hyperCalcaemia, Renal insufficiency, Anemia and Bone lesions).
The new criteria for the diagnosis of MM now include clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma with one or more of the following MDE’s:
- CRAB features, or:
- Serum involved or uninvolved free light chain* ratio of 100 or greater.
- 60% or more of clonal plasma cells in the bone marrow.
- More than one focal lesion on MRI that is at least 5 mm or greater in size.
*Involved free light chain must be ≥100mg/L (all FLC values are based on the Freelite assay)
The IMWG recommends the implementation of these criteria in routine practice and in future clinical trials.
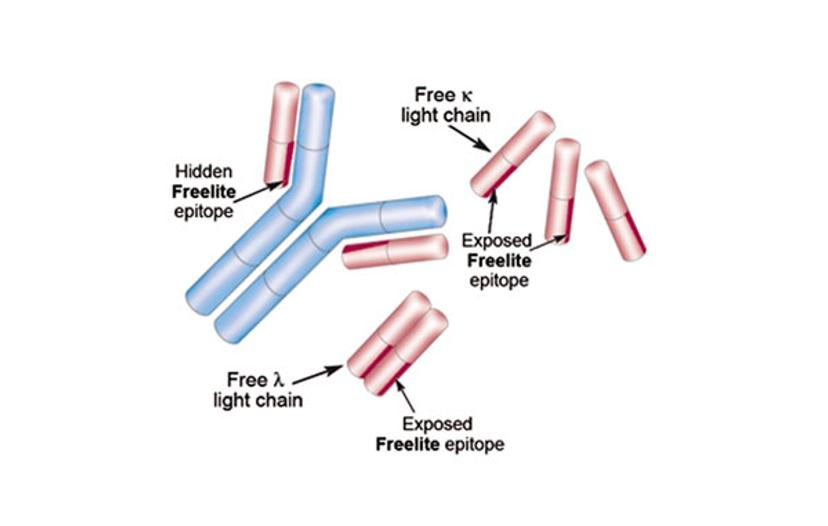
The IMWG guidelines recommend The Binding Site's Freelite product, for free light chain measurement
These international guidelines are now starting to be incorporated into national guidelines. This includes the recently revised 2015 Chinese Myeloma Guidelines, which combine the guidelines from the IMWG, along with the World Health Organization (WHO), and National Comprehensive Cancer Network (NCCN), in listing Freelite by name for free light chain measurement and say that “the technique from The Binding Site Group (Birmingham, UK) is recommended”.
For more information you can listen to Dr. Dispenzieri, Professor of Medicine in the Division of Hematology at the Mayo Clinic discussing the IMWG guidelines here.
