Volumetric dried blood sampling in a patient and lab friendly device – A game-changer for DBS diagnostics
Learn how quantitative DBS technology could transform the clinical utility of dried blood spots and help drive the shift to self-sampling in healthcare
30 Nov 2023
Since its introduction in the 1960s, dried blood spot (DBS) sampling has become a routine practice for newborn screening (NBS) and monitoring of patients with metabolic disorders worldwide. Based on the collection of a few drops of blood on filter paper, DBS methodology offers a convenient alternative to venipuncture that is minimally invasive, cost-effective, and potentially suitable for home- or even self-sampling. Nevertheless, conventional DBS cards have a major limitation in that they lack volumetric control, an issue exacerbated by variation in blood viscosity associated with individual hematocrit levels. As a result, conventional DBS cards are restricted to qualitative or semi-quantitative analyses, while most clinical tests require quantitative results.
Recently, there has been a push to overcome this limitation through the development of devices that can generate a volumetric dried blood sample directly from a nonvolumetric drop of blood. At the forefront of this effort is Capitainer, a Swedish medtech company that aims to drive global access to diagnostics through its novel microfluidics-based DBS technology, quantitative DBS (qDBS). In this SelectScience® interview, we speak with Capitainer's CEO, Christopher Aulin, and two leading experts in the analysis of DBS samples and clinical application of mass spectrometry, Dr. Donald Chace and Dr. Timothy Garrett, to learn more about the capabilities of qDBS and its exciting potential.
Why work with dried blood?
While the collection and storage of liquid blood samples remains routine in clinical settings, DBS microsampling has become increasingly popular in various bioanalytical areas, including disease detection, preclinical animal studies, clinical drug trials, therapeutic drug monitoring, and toxicology. One of the primary advantages of DBS is its ability to minimize the amount of blood needed for testing, making it easier to obtain samples from vulnerable populations, resource-limited areas, and even patients’ homes. Moreover, DBS microsamples can be dried and stored at room temperature, reducing biohazard risks, improving compound stability, and streamlining shipping and transport. “Wet blood poses a biohazard and requires overnight and cold-chain transport, which imposes a huge cost and negates its use for large scale home-sampling, such as population screening,” explains Aulin. “In comparison, dried blood spot samples do not require cold-chain transport, and the removal of water means many analytes remain stable for a much longer time.”
A spot of trouble
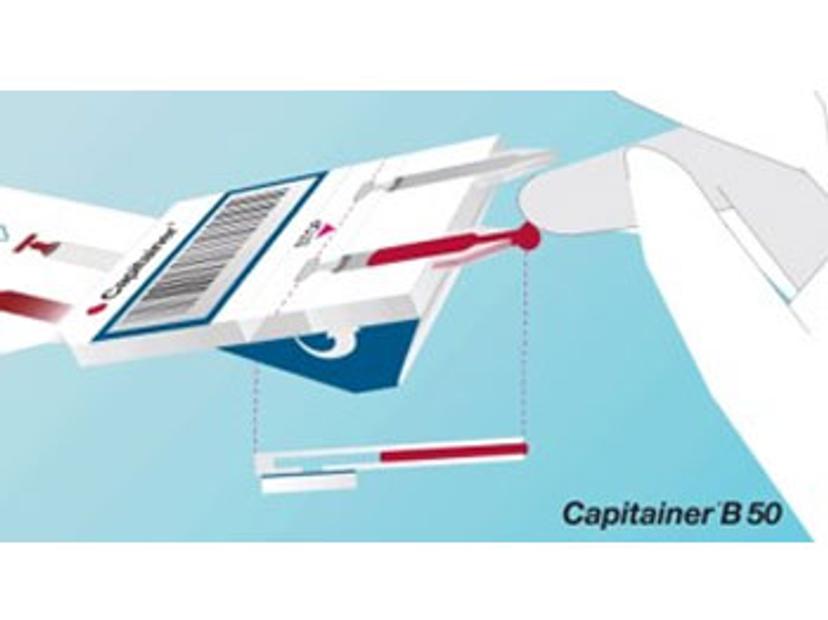
Despite sampling advantages, a persistent barrier to the use of DBS samples for quantitative measurements has been the lack of volumetric control in the blood spots obtained. Here, a major issue is the effect of hematocrit (Hct) on blood viscosity, with higher Hct levels leading to less spreading of blood on filter paper and variability in the volume of blood obtained when a sub-punch of fixed diameter is extracted for analysis. One strategy to overcome the Hct effect is whole-spot analysis with a fixed volume of blood applied to the paper using a capillary or pipette. However, this adds complexity to the method, precluding its use in patient home settings or remote areas.
Introducing qDBS
Born out of research conducted at the KTH Royal Institute of Technology in Stockholm, Capitainer’s solution to this problem relies upon the use of microfluidics and thin water-soluble membranes to meter a fixed volume of blood. After applying blood to the inlet, a metering channel in the device is automatically filled and the blood is transferred to a pre-cut DBS paper disc. Since the entire defined spot is used for analysis, the Hct effect is overcome and the microsample can be used for quantitative analysis.
“From the start, we decided that the product was to be used for quantitative clinical purposes and that the volume precision must be as good or even better than for the standard pipetting of wet blood, which is around 1.5–2.5% CV,” Aulin explains. “Our qDBS devices meet that level of precision, and they have to, as otherwise they would just be screening tools.”
Another major advantage of Capitainer’s devices is their sample protection with the sample embedded in the device without exposure to outer surfaces or physical contact by the sampler. “In contrast to normal DBS paper, everything about these devices was made for home sampling and self-sampling,” adds Aulin. “It’s extremely easy to collect the blood and the cards are placed in a vented pouch until analysis, allowing them to be dried in a protected manner that avoids contamination.”
From newborn screening to beyond
Capitainer qDBS-sampling technology has already been validated in real-world population studies. The technology has been shown to be superior to conventional cards and competitive for use in monitoring patients with phenylketonuria (PKU), a common test used in newborn screening1. As one of the pioneers of the modern-day newborn metabolic screening test that employs tandem mass spectrometry, Dr. Donald Chace is an expert in this field. “Previously, while the accuracy and precision of mass spectrometry solved a lot of issues in newborn screening, the blood spot itself still had a great deal of imprecision,” he says. “This meant you had to lower your decision criteria, which widened the possibility of generating false positives and false negatives.”
With the precision of these cards, a tiny difference is now noticeable, and assays can be significantly improved to reduce the chances of false results.
Dr. Donald Chace
Capitainer
“I spent much of my career working around that challenge, until I came across Capitainer,” he continues. “With the precision of these cards, a tiny difference is now noticeable, and assays can be significantly improved to reduce the chances of false results.”
“There are a thousand different metabolic diseases out there, and currently we’re only utilizing DBS sampling on 50 or 60 of these diseases,” Dr. Chace adds. “Then there are other kinds of diagnostics, beyond inherited disease to acquired disease, infections, and cancer. There are a zillion different things that you can do with DBS, but it all starts with having a better spot that you can develop the assay on.”
Locking in results at the time of collection
In addition to validating the use of qDBS in NBS, Dr. Chace is working alongside Dr. Timothy Garrett, an associate professor at the University of Florida, in collaboration with Capitainer, to expand the use of its cards in other clinical mass spectrometry settings. “Applying mass spectrometry typically involves extensive sample preparation which can introduce errors and be costly for labs to have all the necessary materials,” says Dr. Garrett. “For this reason, one of the areas we’ve been working on is adding reference material to the cards that allow them to be quantitative at the time of collection.”
Here, the team is using stable isotope standards – enriched versions of the compounds of interest that have a slightly higher mass but are otherwise biochemically identical. These standards are added at a precise volume to the cards before distribution and mixed with the blood as it is collected, creating a reference material of known concentration at the time of collection. “Whatever then happens to the analyte in the sample will be paralleled by that standard from the very moment of collection,” explains Dr. Chace. “Even if half of it was degraded, the standard itself would be degraded by the same amount, so the result at the time of collection remains the same.”
Not only can the addition of reference material control the effects of sample transport, preparation, and processing upon analytical results, but the approach could also reduce variation between different labs. “How internal standards are incorporated can vary between labs, and this can lead to differences in the results,” explains Aulin. “If you already have the internal standard together with the blood spot when it arrives at the lab, it's all about the ratio, and any mass spec lab should be able to produce the same result.”
The volumetric precision and unique design of the Capitainer cards are critical to this approach. “You can't generate a known concentration of reference analyte unless the volume of blood you're adding it to is precise,” explains Dr. Chace. “Moreover, as Capitainer cards feature a capillary between the inlet and the paper, the paper can’t be contaminated. This design is extremely important, as the risk of contamination is one of the reasons this approach couldn’t previously be used with conventional DBS.”
“Today, even with liquid blood, there are no equivalent workflows for this approach – it just wouldn’t be feasible with the amount of standard required and how it would need to be added,” he adds. “I think this idea is going to change everything.”
So far, the team has tested these cards for traditional applications such as NBS and is exploring other clinical areas such as metabolomics and lipidomics. “In a 10-microliter sample, we can easily get 1,000 to 2,000 metabolites, and we can use those readouts to measure differences in individuals that may have cancer, diabetes, or other disorders that could be important to measure,” shares Dr. Garrett. “By incorporating reference material, we can enhance the precision of measuring these biomarkers, which could lead to direct clinical applications and ultimately contribute to the improvement of healthcare.”
From one single card, we can get proteins, lipids, and metabolites, and it is possible to imprint the standards for each of those compound classes to conduct a multi-omic analysis.
Dr. Timothy Garrett
University of Florida
Future outlooks
From supporting population-wide studies to enabling multi-omic-based diagnostics from a single microsample, the future implementation of qDBS could transform the landscape of medical diagnostics. “With a precise and convenient card, the number of applications is extraordinary,” Dr. Chase enthuses. “We can do newborn screening, nutrition monitoring in premature babies, monitor the metabolites of individuals throughout their life to help manage diseases, all the way to post-mortem screening. We can also measure the same set of metabolites in pets, in environmental studies, or forensics – there’s almost no limit.”
For me, one of the interesting aspects of the technology is that it represents a unique way to separate all kinds of omics,” adds Dr. Garrett. “From one single card, we can get proteins, lipids, and metabolites, and it is possible to imprint the standards for each of those compound classes to conduct a multi-omic analysis.” Aulin shares this excitement, emphasizing the potential of combining proteomics and metabolomics analyses to rule out diseases such as cancer and optimize healthcare resource allocation. “Diagnostics have incredible power in optimizing healthcare and reducing the social cost,” he says. “Using this technology, we could start with just a few compounds or diseases and build out from there.”
“Robert Guthrie started in 1960 with one DBS assay for one marker, phenylalanine for one rare disease, PKU,” adds Dr. Chace. “Just look where we are now,” he concludes.
References
Carling, R.S., Emmett, E.C. and Moat, S.J., 2022. Evaluation of volumetric blood collection devices for the measurement of phenylalanine and tyrosine to monitor patients with phenylketonuria. Clinica Chimica Acta, 535, pp.157–166.
