‘We Save Hours of Work Daily by Streamlining Our Clinical LC-MS Workflow’
How data management solutions are alleviating bottlenecks in the clinical laboratory and saving hours of analyst time, every day
26 Jun 2018

In the clinical laboratory, workflow efficiency is a key to maintaining patient safety. Recently, great strides have been made in the technology available to clinical scientists that streamline many medical laboratory processes, particularly in the field of separation science.
At the University of California San Diego, Dr. Judy Stone, a senior clinical laboratory specialist within the Center for Advanced Laboratory Medicine, spends much of her time focused on method development for clinical sample analysis using liquid chromatography tandem mass spectrometry (LC-MS/MS). Here, SelectScience speaks to Stone, to understand how her lab has leveraged advances in data management solutions to save hours of work each day.
SS: Please give a brief overview of your background, and what your current role at the University of California San Diego entails
JS: I’m what would be described in the US as a clinical chemist; I started out working in a clinical laboratory as a bench technologist and then I went back to graduate school, gaining a Ph.D. in clinical biochemistry followed by a postdoctoral fellowship in the same topic. Since then, the vast majority of my time has been spent working in hospital laboratories as a laboratory director or R&D scientist.
I have been working in separation sciences specifically since 1984, and with mass spectrometry intensively from 2000. My current role for UCSD involves high complexity patient testing, where I am largely responsible for method development and validation with our mass spectrometry platforms. A great deal of what I do encompasses troubleshooting and improving processes to obtain the same quality of testing, in a way that reduces cost per test. That’s how I became involved in exploring information management; there is so much to be gained in terms of workflow efficiency here.
SS: With your experience in the separation sciences, what has been the most paradigm-changing development in technology across your career?
JS: The biggest shift I’ve seen throughout my career has been the emergence of LC-MS as a viable technology in the clinical laboratory. This has made it feasible to do high-quality quantitation in larger volumes with less sample preparation. Furthermore, the lower detection limits of these instruments have made it possible to analyze a broader range of substances, without derivatization of our samples. The coupling of liquid chromatography with mass spectrometry, along with the advance of triple quadruple mass analysis for unparalleled quantitation of substances in samples, has really been the game changer in the field.
SS: How has this technology benefited the work that is conducted in your laboratory at UCSD?
The software saves us hours of analyst time a day
When working with large volumes of clinical samples, LC-MS/MS is economically beneficial and provides unequalled substance specificity. For example, we’re able to discriminate between 0.5ng/ml and 1.0ng/ml of drug substance in a patient sample, and characterize the exact agent in their system.
It has become very clinically meaningful to use LC-MS/MS for therapeutic compliance in pain management testing, abstinence in substance abuse testing, and many labs are now turning towards therapeutic drug monitoring with LC-MS. Particularly for experimental compounds, it’s important to determine whether a patient isn’t responding because the drug isn’t present, or whether they’re not responding despite its presence within the therapeutic range. Essentially, there is no technology that can rival LC-MS/MS in this field, so it is well embedded at UCSD for these types of applications. Another big driver for investing in this technology has been for greater specificity and sensitivity than automated immunoassay for vitamin, metabolite and hormone testing, the latter particularly in women and children.
However, there remain many clinical laboratories lacking this technology in their special chemistry and toxicology testing compendium. Whilst the procedure is complex, this can be addressed with training, something I’m very passionate about and heavily involved with.
SS: Talk me through the LC-MS/MS workflow in your laboratory, right from sample preparation through to data management
JS: Samples come in, and are automatically sorted and accessioned. We organize them into our batch testing by inputting samples into the LIS, laboratory information system, in the order we test them in. Following automated sample preparation, data is transferred from the liquid handler plate map of sample IDs to the mass spectrometer instrument; all the user must do is import this information into the machine.
Once this is all completed in the morning, samples go onto the Waters ACQUITY™ UPLC™ I-Class/ Xevo™ TQ-S IVD System early in the afternoon. The instrument is then running for 18 or 19 hours and we report the data the following morning.
The great thing about our instrument is that it allows data to be transferred electronically to the LIS, using Waters’ specialized data management software. We also have auto-verification rules set up so that after an analyst checks that the peaks are correct, the batch is sent across the interface, and results are evaluated automatically by software against reportable range, LC-MS metadata criteria, and patient demographics for each sample to make sure it passes. The information is then immediately available to the physician. The only manual involvement is when samples fail, and our scientists look up the exceptions and after review, accept or schedule for retesting.

SS: Please explain in a bit more detail which types of software you use and how they address some of the challenges or bottlenecks in the workflow
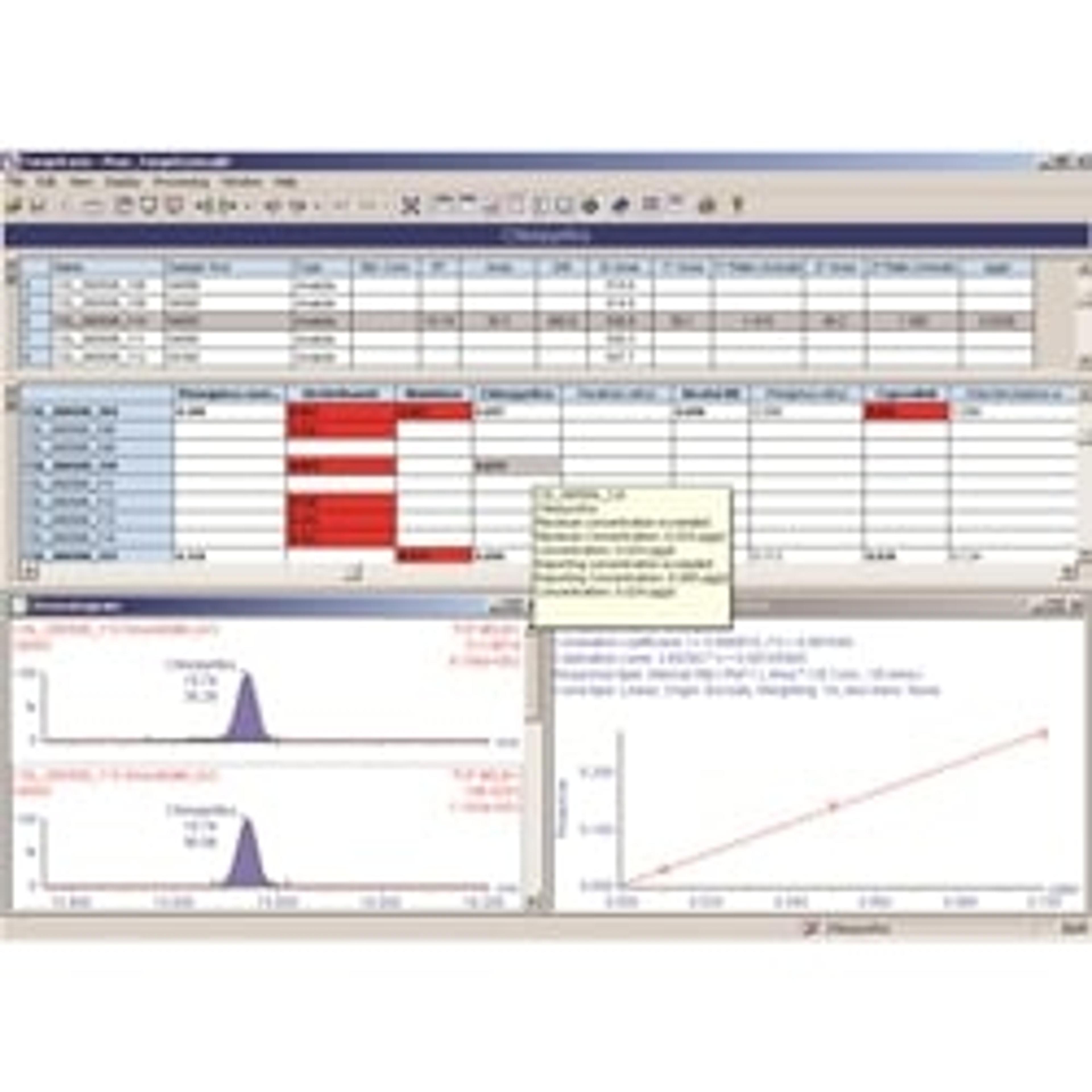
JS: Sample set-up and data analysis are the two main challenges to tackle in clinical mass spectrometry workflows. They’re both complicated and often involve working with more than one piece of software, but focusing on effective data management has been significant in terms of saving us analyst time. We use the Waters MassLynx™ (IVD) Software to run the instruments and TargetLynx™ Application Manager to run the data analysis. Within TargetLynx there is what Waters call their LIMS HL7 enabled interface driver, which we worked with them closely to implement at UCSD. Currently, we use our interfaces unidirectionally, uploading the results directly from the platform to the LIS.
With this capability, one person can analyze 100s of samples a day, especially considering all the data management provisions within TargetLynx. To identify samples that require additional analyst review, the user has the ability to select from a long menu of parameters that describe the peaks: peak width, peak area to height ratio, percentage deviation, and many more. If you put all our batches together, the software, rather than a human being, saves us hours of analyst time a day in finding the exceptions in a batch of more than 100 samples.
SS: I understand you do a lot of work in method development, improving the robustness of workflows, as well as developing new ones. How are you looking to improve your workflow efficiency even further in the future?

JS: We certainly have interest in moving towards bidirectional interfacing within our data management. What we would like to do, is have key clinical information available to our liquid handler that enables discrimination for individualized sample preparation, based on immunoassay results and patient gender for example. I firmly believe the biggest potential lies in information management, for not only saving time, but also increasing the reach of this technology.
We would also like to achieve more routine multiplexing on our platforms; running the same application in two columns in parallel would reduce the testing time by half. If we could automate the process of preparing samples that exceed the dynamic range of the instrument that would be fantastic, and furthermore, random access testing, rather than calibrating with every batch, would dramatically reduce analyst workload in our lab.
However, when you’re doing this kind of innovative work with analytical platforms like LC-MS/MS and instrument to instrument, instrument to LIS interfaces they all have to be intensively validated. So it can be complicated, expensive and time-consuming but I’ve had a very positive experience overall with Waters. In the long run – the investment in more sophisticated information management has paid off in higher productivity per analyst. Waters are constantly looking for ways to facilitate our workflow, so I look forward to working with them in the future.
Find out more about Waters' family of LC-MS/MS in vitro diagnostic medical devices