Webinar Highlights: Overcoming Sample Prep Challenges in Clinical Research
Phenomenex’s sample preparation product manager shares his expert advice on preparing challenging biological sample matrices for LC-MS/MS
7 Nov 2017
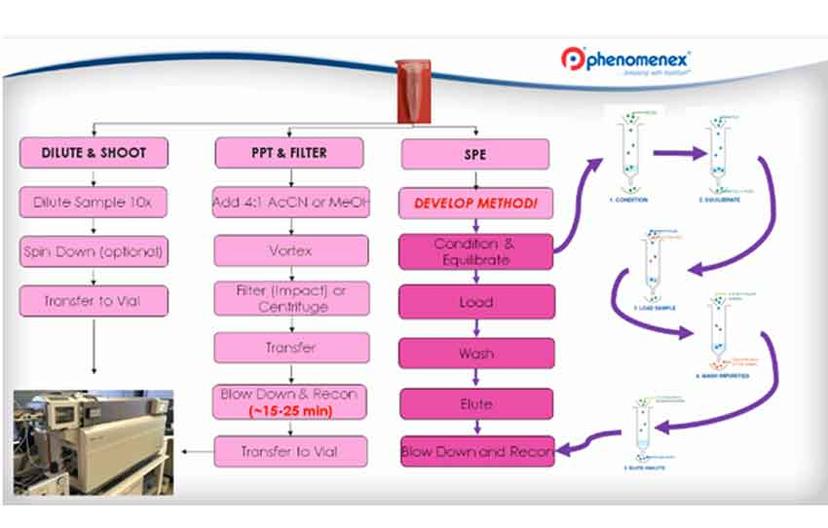
In this recent webinar, Matt Brusius, Sample Preparation Product Manager at Phenomenex, presented a comprehensive guide to method development for difficult analytes and matrices. He also presented three case studies covering specific sample preparation challenges and how to overcome them. In clinical research it is important to prepare samples for LC-MS/MS analysis. Preparing some biological samples can be difficult and time consuming. However, preparing these samples improves the quality of chromatography, concentrates the analyte of interest and switches the analyte into a better solvent for analysis.

Read on for highlights from the webinar Q&A session or watch the webinar on demand.
If ß-Gone is a shorter and easier protocol with better sensitivity for a full panel, why would I consider SPE as a sample prep option?
MB: I would consider SPE as an option as it will give you a cleaner sample. It depends on how much time you have for method development and what is important to you. If you want the absolute cleanest sample, and you want the peace of mind that you are going to get 20,000 injections in your column, SPE can provide peace of mind for you in comparison to ß-Gone. ß-Gone is a step above the protein precipitations and dilute and shoots of the world, but if you have the time to develop the SPE method, it’s always going to be cleaner. You can also concentrate up your sample – you can’t actually concentrate your sample with the ß-Gone phase, it’s a chemical filter, there is no concentration effect, whereas SPE has a concentrating effect, if you’re doing GC-MS you have to do SPE to hit the detection limit.
Does the Phree phospholipid removal product also do SPE? Would there be any need to do additional SPE?
MB: Not typically. That’s not really the goal of doing phospholipid removal. Phree can be helpful as a way of replacing SPE. You could do it before SPE, but I think it would be overkill. It really depends on the SPE method – are you doing a large panel? How much are you washing with? How effective is your SPE method at removing phospholipids? If you’re doing protein precipitation followed by SPE, for example, and your SPE step for the sample is only a 5% wash, there’s a good chance that if you’re only doing a 5% wash and you’re eluting off with 100% organic solvent you’re going to catch the phospholipids onto your cartridge and then elute them back off. So, if SPE method doesn’t have a lot of washing to it, using a phospholipid removal device beforehand could be useful. I would personally just try to use the phospholipid removal device as a standalone option but you can stack them if you want to, depending on what the other stipulations are.
What kind of suggestions do you have on removing surfactant from samples?
MB: Surfactants can be really difficult. Often, they require a very specific amount of washing. I’m not sure of the context of the question – oral fluid collection devices contain a significant amount of surfactant. If you’re able to do an SPE with them, then an ethyl acetate wash usually does a pretty good job of removing most of the surfactant. That’s one way you can get them out of your sample. If you’re doing full blown SPE, if there are other problems with or other things associated with it – I’d have to look at that method in particular. But ethyl acetate usually does a good job of solubilizing the surfactant enabling it to be removed prior to elution.
Watch the full webinar on demand or find out more about Phenomenex’s sample preparation solutions
Do you use any of the technologies mentioned in this webinar? Write a review today for your chance to win an Amazon voucher worth $400 or an iPad.