Webinar Highlights – Real-Time Live-Cell Analysis for Immunologists
Dr. Del Trezise answers your questions about using real-time cell imaging to gain deeper insights into immune cells
18 Apr 2017
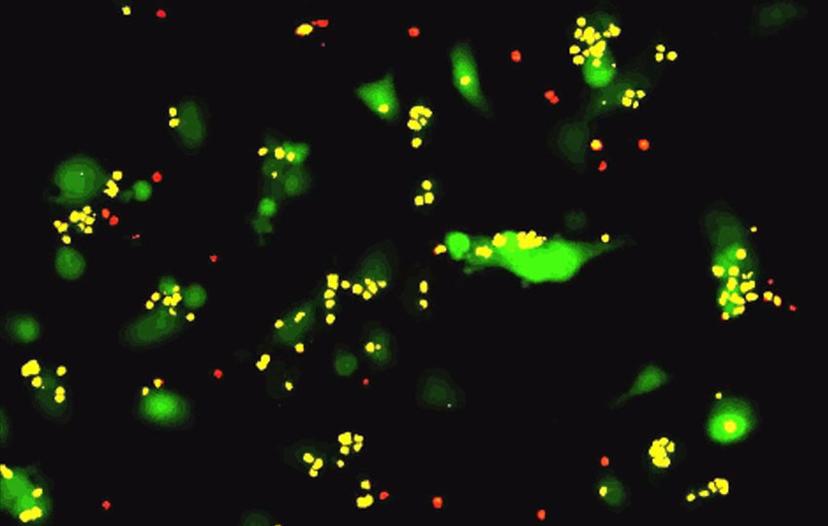
T-cell killing assay imaged using the IncuCyte System. Image courtesy of Mauro Castellarin, Ph.D., University of Pennsylvania.
In his recent webinar, Dr Del Trezise, Senior Scientific Officer, Co-founder and Director of Essen BioScience Ltd, discussed the advantages of using real-time live-cell analysis in immunology. He presented several case studies to demonstrate how the IncuCyte System could be used for kinetic quantification and functional analysis of various types of immune cell assays.
Read on for highlights from the Q&A session with Dr Trezise, or if you missed it, watch the webinar on-demand.
Q: You mentioned plate coating for suspension cell assays, why do you need a coating and which coatings would you recommend?
A: Without a plate coating, there is a tendency for cells to disappear out to the edges of the well and this makes them harder to focus on. We recommend coating wells with poly-l-ornithine. Poly-l-ornithine is an inert synthetic amino acid chain with positively charged groups which is commonly used for promoting cell adherence. Coating with Poly-L-ornithine does not interfere with the biology of the immune cells, but provides sufficient adherence to prevent them from disappearing to the edge of the well.
Q: Can you measure cellular engulfment using the IncuCyte?
A: Yes, absolutely. For measuring engulfment of cells, or efferocytosis, we use the same principle as that used for measuring engulfment of bioparticles, except you’ll need to label the cells with pHrodo. You begin by labelling the target cell, for example an apoptotic neutrophil, and then mix the labelled target cells with the effector cells, for example macrophages. As your target cells are engulfed, you’ll see the appearance of fluorescent signals as the labeled cells are internalized into the acidic phagolysosome. We sell pHrodo labelling kits to make it easy for you to label your target cells.
Q: Can the device run several separate experiments or plates simultaneously and can they be put into the device at staggered times?
A: Yes, but with some constraints. The IncuCyte system is provided with 3 different objectives and at any given time you can only run experiments with one objective. You can’t swap objectives between one plate and another within a system. However, if you do all your experiments using the same objective, you can run multiple experiments at the same time. For example, you can run a phagocytosis experiment alongside an apoptosis experiment, alongside a chemotaxis experiment.
Another thing that you’d need to agree on with your other colleagues using the machine is the duty time. You wouldn’t have to start all the experiments at the same time, but you would need to have them on a compatible duty cycle, for example set to take one image every two hours over each of the plates.
So, there is a degree of flexibility which allows you to conduct multiple parallel experiments, but you can’t mix and match. This is to maintain a level of simplicity to make it easy to set up and control the instrument.
Q: Can the device follow the time course of cell interactions over long periods of time?
A: Yes, we’ve been able to run assays on the IncuCyte for several months with some cell types.
As with all cell culture experiments, over time the cells will deplete nutrients and excrete toxic metabolites, so you will need to refresh the media. You’d probably need to refresh the media once a week or so. To do this, you’d just need to remove the culture from the IncuCyte, refresh the media and then replace the culture in the same position and restart the software to continue acquiring data. At the end of the experiment, the software will concatenate the pre- and post- media refresh data, so that when it’s summarized, it will be seamlessly connected.
The forte of the IncuCyte is that, apart from when refreshing the media, everything stays within the incubator so the cells can be maintained for longer, making long-term assays possible.
Q: What size cell sample is required to make a good observation of macrophage chemotaxis?
A: One of the advantages of the IncuCyte chemotaxis solution is the ability to work with much fewer cells. We typically run our chemotaxis macrophage assay in the range of 1000 to 2000 cells per well. But in other formats such as classic or Boyden chamber assays and chamber, or transwells, you’d typically need between 50,000 to 100,000 cells per well.
If you have precious cells which are difficult to obtain and low in cell number, then this format is very amendable to maximizing the amount of information you can glean from a low cell number.
Q: When carrying out proliferation assays, what happens to the linear relationship once 100% confluence is reached?
A: We can’t reliably quantify the proliferation of the cells once they reach beyond 80-85% confluence by using the IncuCyte phase confluence metric. As the cells begin to pack in and grow on top of each other the relationship between cell area and cell number begins to break down.For measuring cell proliferation in high confluence cultures, you would need to use our other method based on nuclear labeling of cells. This allows you to resolve the true cell count by counting the number of nuclei, as opposed to just looking at the occupied area using the phase metric.
Watch the full webinar on-demand, or find out more about the IncuCyte® Live Cell Analysis System.
Do you use any of the technologies mentioned in this webinar? Write a review today for your chance to win an Amazon voucher worth $400 or an iPad Air®.

