Win-win collaboration delivers pharma efficiencies, data compliance and weighing excellence
Dr. Andreas Schüler and Sebastian Weber discuss how a pioneering Sartorius and Abbott Informatics has produced cutting-edge weighing technology to make scientists’ lives easier
4 Oct 2020

With time a precious commodity and ever-increasing regulatory scrutiny, pharmaceutical laboratories are always on the lookout for simple solutions to make their routine tasks easier. Here, we speak with experts Dr. Andreas Schüler, technical sales support manager at Abbott Informatics, and Sebastian Weber, product manager at Sartorius, to find out how they are working together to help scientists weigh samples in the most efficient yet trusted way through integration of the STARLIMS solution and Sartorius Cubis® II into one simple platform designed to increase lab efficiency, save time and overcome compliance challenges.
Tell us about the collaboration between Sartorius and Abbott Informatics and how it is benefiting scientists
AS: Sartorius and Abbott Informatics have a lot of overlap in our customer bases. We have many scientists that have to use a Sartorius balance and also our STARLIMS solution in their daily work. Based on that, it made a lot of sense for us to collaborate and investigate together how we can design a means of integrating our products in a way that makes the lives of our users easier.
The big picture goal is we want to make use of RESTful web services technology to design a LIMS balance integration that is on the one hand easier to set up than the traditional means of integrating balances with a LIMS, but also fully compliant with the highest standards of data integrity and IT security. The modern web service-based technology allows us to design something that is objectively better than the traditional way of setting up these balance LIMS integrations.
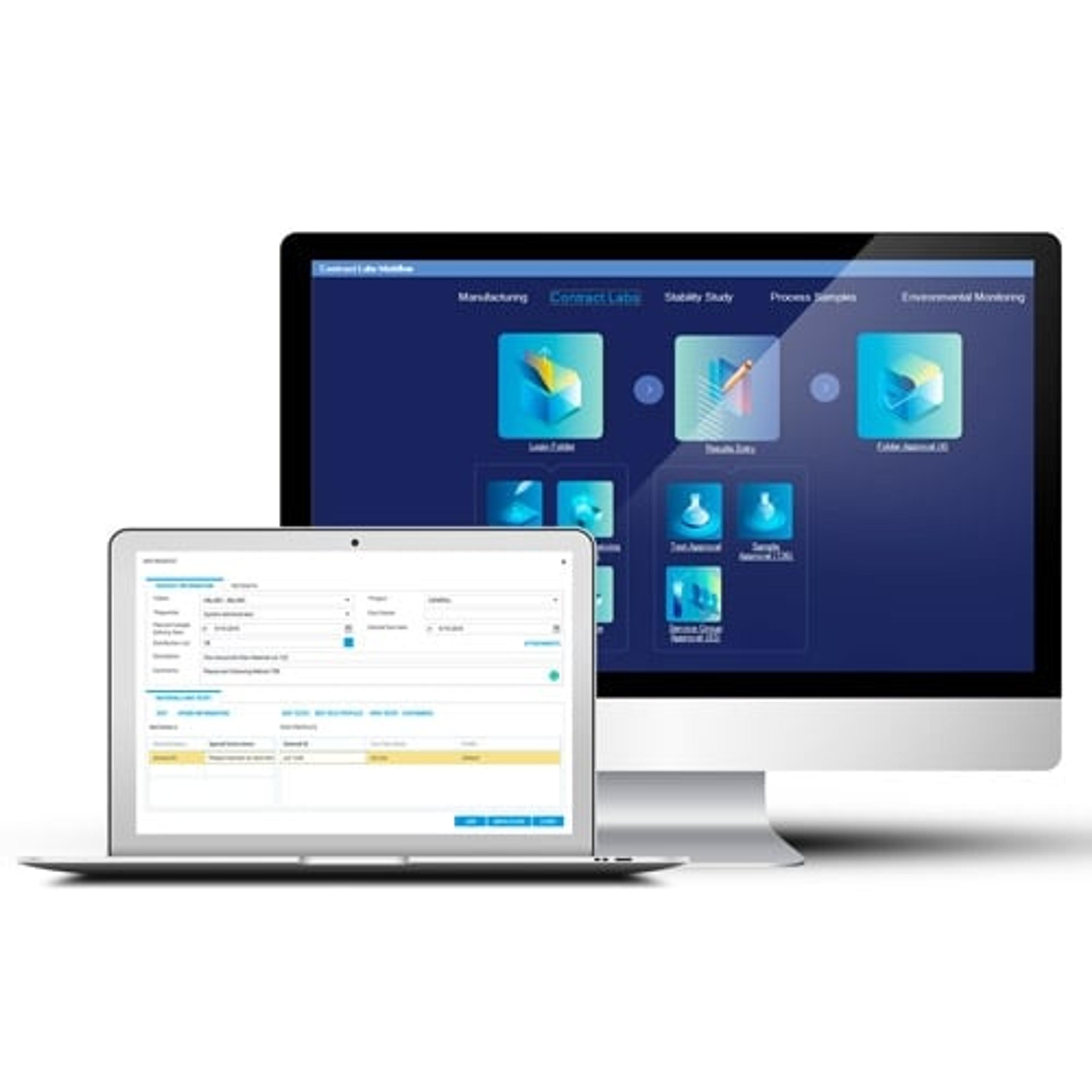
How does the Cubis II integrate within STARLIMS?
SW: Every system has its own focus in this solution. STARLIMS offers an outstanding LIMS for laboratory workflow and data management, with the Cubis II balance we focus on a simple, efficient and safe weighing process for trusted results. The goal was to integrate the Cubis II balance over standard IT protocols into STARLIMS, without the need of a converter or additional IT software. In this way, we ensure an intuitive and seamless operation and automated data transfer at the highest level of security.
Sample lists are transferred from STARLIMS via Ethernet or WIFI to the balance so that the entire weighing process can be controlled directly on the balance screen. This eliminates the need to use a computer next to the balance in the laboratory, an area where space is very limited. After weighing, the results including the necessary metadata are automatically transferred back to STARLIMS, which prevents errors in manual data entry. I would like to mention that we did not only pay attention to the pure transmission of the weighing results, we designed the whole process in the safest and most efficient way. For example, before starting the weighing process on the balance, Cubis II authorizes the user against STARLIMS and checks its own device status in the STARLIMS inventory management, e.g., to avoid working with an uncalibrated balance.
How does this integration increase lab efficiency for users?
AS: When information is sent to the balance, it would then guide the user through the process and tell them what to weigh in and what the current result is supposed to be. This, therefore, ensures the user follows a standard operating procedure (SOP). It can also be used for routine activities such as daily calibration, particularly regarding the calibration of LIMS, and when this is executed, we can keep track of the service steps of the equipment. We can keep track of whether it was calibrated properly on a particular day and tell our users on the balance side if this equipment is good for use or not. Automatically keeping track of whether the equipment was calibrated or not improves efficiency.
What challenges are your customers facing and how does your technology help overcome them?
SW: We address two important points: data integrity and connectivity between LIMS/ELN and laboratory instruments. The integrity of data is essential in regulated industries and comes especially into focus during regulatory audits. Paperless documentation and digital workflows can avoid data integrity violations and increase efficiency in daily laboratory work.
The biggest challenge for this kind of digitization is the non-existent standard for the integration of instruments into a LIMS/ELN. The integration is often only very minimalistic via a serial connection, complex middleware, or the parsing of report files. This rarely fulfills all data integrity requirements unless a specific island from the laboratory instrument provider is built, which is very complex and costly.
With Abbott Informatics, we have found a partner who, like us, offers an open system architecture for flexible and future-proofed connectivity options based on common IT standards like RESTful web services or LDAP. This allows us a simple but comprehensive integration, without the use of vendor-specific protocols or large system adaptations on both sides.
How are the demands on data integrity, IT security and compliance met?
SW: We strictly follow the ALCOA principles to meet the regulatory requirements such as FDA 21 CFR Part 11 or EU Annex 11. STARLIMS and Cubis II are designed in a way to provide all necessary functionalities, such as role-based user management or audit trail, for regulatory compliance out of the box. Therefore, it was simply to develop a comprehensive integrated solution based on the existing features.
Both companies also pay a lot of attention to the field of IT security during product design. That is the reason why only encrypted and proven IT protocols such as HTTPS were used.
What are the key standout features of this integrated system?
AS: We’ve designed an integration that allows a plug-and-play kind of setup which is easier to implement than the traditional means of balance integration was, and simultaneously we’re still ensuring the highest possible levels of data integrity over the entire life cycle. That I would say is the key standout feature.
What do you see as the future developments in this area?
AS: I think Sartorius has really set a good example here and I think this is an example that many other instrument vendors are going to follow in the next couple of years because the technology we are using here, there’s nothing proprietary about this. These are protocols and technologies that any company can use for their devices and I believe many instrument vendors will follow this example and offer comparable solutions for integrations into LIMS systems.
What that means for the users, what that means for laboratories, is that there’s a real possibility for the IT landscapes in the laboratories to become simpler and easier to maintain, because if you allow the systems to directly communicate with each other like that, you reduce the need for third-party tools. They can directly speak to each other and this leads to a simpler landscape that’s easier to set up and easier to maintain.
SW: Digital transformation is probably one of the hottest topics in the laboratory right now. An important key factor of digitalization is the direct integration of instruments into a LIMS to improve data integrity, increase productivity and use advanced analytics capabilities. With our solution, we have shown how a full integration can be implemented without the need for middleware or converters. This simplifies the usage for the operator, as well as the IT administration and qualification part.
From the Sartorius perspective, the future is to integrate more instruments on the same level as Cubis II to prevent data integrity problems and simplify processes
Do you use Abbott Informatics or Sartorius products in your lab? Write a review today for your chance to win a $400 Amazon gift card>>