News & Articles
Selected Filters:
Beckman Coulter Diagnostics Announces Strategic Collaboration with UCI
Agreement to focus on accelerating campus innovation from lab to real world via UCI Applied Innovation
Pivotal Study Reveals Effectiveness of Blood Tests in Screening for Sleep Apnea in Adult Males
Recent findings show initial testing for three specific biomarkers may provide superior results over standard screening methods
Beckman Coulter Diagnostics Receives U.S. FDA 510(k) Clearance for High-Sensitivity Troponin I Assay
First high-sensitivity troponin I assay available in the United States enables clinicians to provide improvements in cardiac patient management
Beckman Coulter Files a 510(k) Submission with the FDA for its Early Sepsis Indicator
Offered as part of a routine CBC with differential test, the hematology-based solution is intended to alert emergency department clinicians to sepsis or the possibility of sepsis earlier than any other indicator
New Hematology Analyzer Software to Help Laboratories Deliver Quality Results
Beckman Coulter Diagnostics launches software version 2 - enhancements improve flagging and streamline the quality control processes
Beckman Coulter Launches the DxH 900 Hematology Analyzer
The new system aims to deliver fast, accurate results through near native-state cellular characterization, precise flagging and a 93% first-pass throughput
Beckman Coulter Diagnostics Announces CE Mark of DxH 520 Hematology Analyzer
The robust, easy-to-use system aims to boost patient care by combining many of the analytic strengths of a larger-volume instrument with the ease of a table-top analyzer
Simple Blood Test Enables More Informed Prostate Cancer Biopsy Decisions
Prostate health index (phi) test to be exhibited at the AUA annual meeting in San Francisco
Beckman Coulter Achieves CE Mark for Early Sepsis Indicator
First-of-its-kind hematology-based test intended to alert emergency department clinicians to the risk of developing sepsis
Beckman Coulter Diagnostics Announces Global Commercialization of Access Sensitive Estradiol
Access Sensitive Estradiol assay aims to provide a better operational efficiency while delivering quality results
Improve Your Material Analysis with New Next-Generation Laser Diffraction Particle Analyzer
Beckman Coulter Life Sciences' new LS 13 320 XR delivers fast, accurate and reproducible particle analysis data
Beckman Coulter Life Sciences Launches New Family of FFPE Reagent Kits
Single chemistry system extracts total nucleic acid, DNA and/or RNA from single FFPE tissue sample
Beckman Coulter Diagnostics Adds 6-Acetylmorphine (6-AM) and Buprenorphine (BUP) Assays to Comprehensive Chemistry Test Menu
Clinical laboratories gain greater confidence in opioid testing with proven high-specificity and high-sensitivity
Beckman Coulter Diagnostics Receives FDA Clearance for the Automated Access AMH Immunoassay to Benefit Fertility Patients
U.S. labs now able to use the automated Anti-Müllerian Hormone (AMH) assay with the access 2 or DxI Immunoassay systems
Beckman Coulter’s New DxONE Workflow Manager Enables Lowvolume Laboratories to Improve Turnaround Time and Maximize Staff Efficiency
A new cost-effective, cloud-based middleware solution helps low-volume laboratories streamline processes and minimize costs
Significant Reduction in Biopsy Procedures Performed when Prostate Health Index Testing is Used Routinely by Physicians
Multi-center observational study demonstrated a 24% reduction in biopsy procedures performed for men tested with phi compared to historical practice
Beckman Coulter Diagnostics Receives CE Mark Clearance for its High-Sensitivity Access hsTnI Assay
New assay enables hospitals to provide efficiency improvements in cardiac patient management
Beckman Coulter Hosts Flow Cytometry Educational Programme for Diagnostics and Research Applications in the Clinical Setting
Beckman Coulter to present at the European Society for Clinical Cell analysis (ESCCA) 2017
Beckman Coulter Life Sciences Showcases Clinical Flow Cytometry Innovation at AACC 2017
New Products Address Diagnostic Challenges and Workflow Requirements
Monitor Multiple Instruments in Real-Time with DxONE™ Clinical Information Management Tools
This informatics portfolio provides integrated solutions empowering operational and clinical decision-making
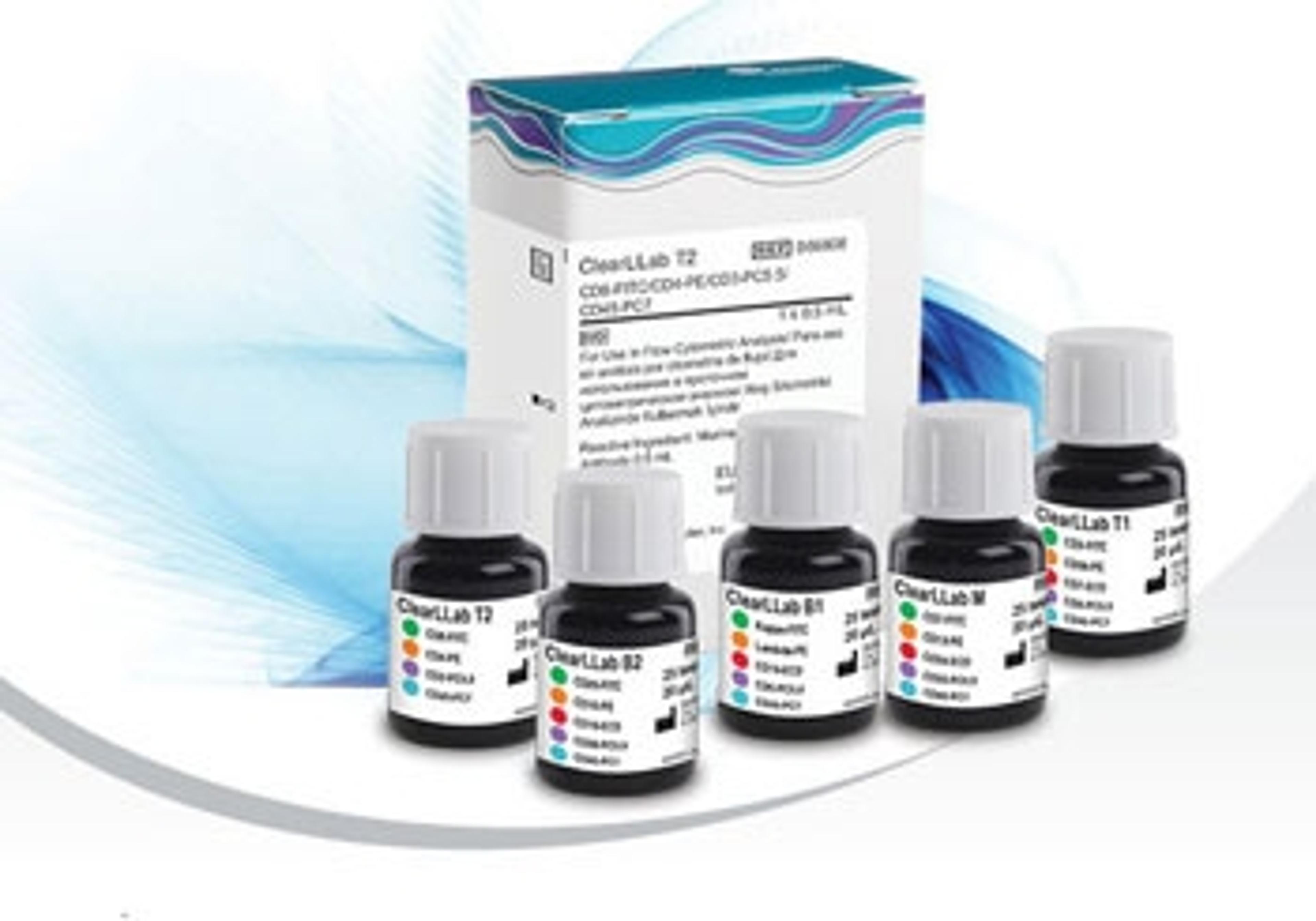
Beckman Coulter Receives FDA Clearance for First IVD Test Delivering Flow Cytometric Leukemia & Lymphoma Analysis
ClearLLab products improve laboratory workflow by reducing manual preparation and routine validation