News & Articles
Selected Filters:
World-class speaker lineup announced for Virtual Neuroscience Summit 2020
Join thousands of scientists from around the world as they come together online to discuss the hottest topics and latest technology solutions in the field
Study shows early treatment for leg ulcers leads to better outcomes for patients
Early surgical treatment of leg ulcers caused by varicose veins improves healing and reduces the risk of the condition coming back
QIAGEN acquires NeuMoDx Molecular, Inc., rounding out portfolio of PCR-based diagnostic automation systems
Menu of test solutions for infectious diseases – which already includes COVID-19 – to be expanded, especially in the U.S.
Promega developing OncoMate MSI Assay as companion diagnostic for endometrial cancer drug candidate
Promega and Incyte intend to work together in the future to develop the Promega OncoMate MSI Assay as a companion diagnostic
DiaSorin's Simplexa COVID-19 direct molecular test CE marked for saliva specimens
The Direct assay is validated on a wide range of specimen types, and can be run in parallel with the Simplexa Flu A/B & RSV Direct Gen II assay
Innatoss Laboratories launches neutralizing antibody public testing service for SARS-CoV-2 in Europe using GenScript's cPass kit
cPass is first commercially available product to rapidly detect neutralizing antibodies capable of eliminating virus
Agilent expands line of flow cytometers with the NovoCyte Penteon
The new line aims to provide unmatched sensitivity, resolution, speed, and flexibility of colors
Research shows that stroke scans could reveal COVID-19 infection
Emergency scans captured images of the top of the lungs where a fluffiness known as ‘ground glass opacification’ allowed COVID-19 to be diagnosed
AstraZeneca acquires oral PCSK9 inhibitor program from Dogma Therapeutics
AstraZeneca anticipates entering clinical development in 2021
Beckman Coulter praised by Frost & Sullivan for accelerating sepsis detection with the DxH 690T
The technology is said to be superior at identifying cell types in human blood, providing faster and more reliable assessments for clinical decision making
Roche launches quantitative antibody test to measure SARS-CoV-2 antibodies
The new Elecsys Anti-SARS-CoV-2 S test can quantitatively measure the level of antibodies against SARS-CoV-2 in patients who have been exposed to the virus
Bio-Techne, QIAGEN expand partnership on exosome technology
The partnership aims to co-market exosome technology as well as the joint development of new exosome based products
Siemens collaborates with CDC to develop a novel process for standardizing SARS-CoV-2 assays
The collaboration aims to define threshold for neutralizing antibody sufficient to confer immunity
QIAGEN to launch rapid portable test that can analyze over 30 samples per hour for SARS-CoV-2 antigen
Access Anti-SARS-CoV-2 Antigen Test, developed in collaboration with Ellume, aims to provide accurate results in less than 15 minutes
Thermo Fisher announces collaborations to meet unmet clinical needs in biomarker discovery and characterization
Agreements with AstraZeneca and the University of Nebraska Medical Center showcase new workflows to improve the throughput, robustness and standardization of clinical biomarker analysis
CombiGene and Cobra Biologics sign agreement to secure GMP production of plasmids
The agreement is part of preparations for GMP production of material for the first clinical study of CG01
Important considerations for choosing a SARS-CoV-2 assay: Your questions answered
Watch this on-demand webinar to discover the critical factors when choosing an assay for coronavirus testing
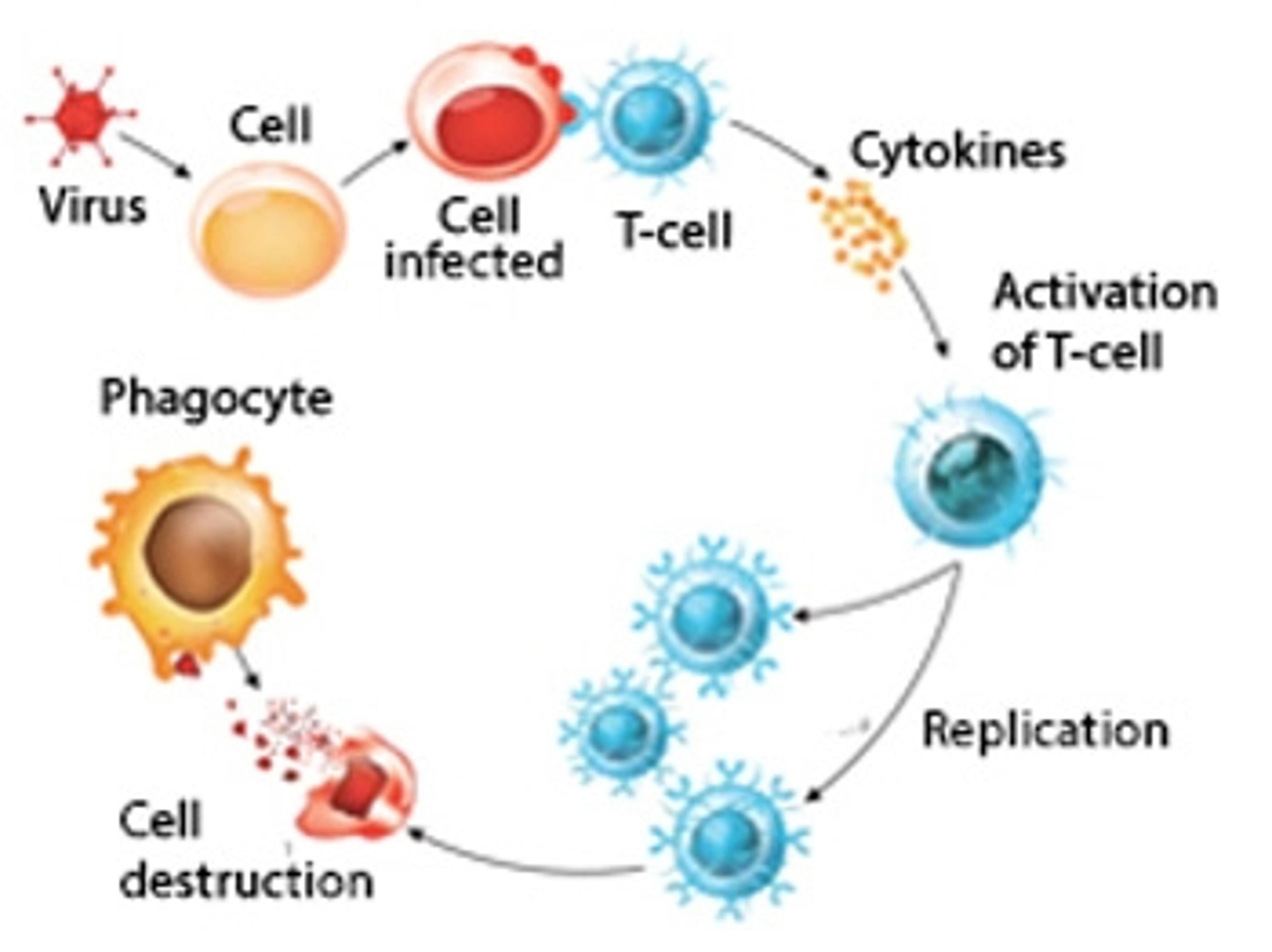
Persistent immune memory of COVID-19 found in recovered patient T cells
New research published in Nature Immunology show strong T cell responses in patients recovered from COVID-19
FDA approves first NGS-based companion diagnostic for RET fusion-positive non-small cell lung cancer
CDx approval expands clinical utility of Oncomine Dx Target Test to identify candidates for GAVRETO
Roche granted FDA clearance for BK virus test to support better care for transplant patients
New test expands Roche molecular test menu for transplant patients, enabling simultaneous testing of BK virus with Cytomegalovirus and Epstein-Barr virus
DiaSorin partners with MeMed to develop and commercialize diagnostics solution
Test to be integrated in over 5,000 DiaSorin analyzers, allowing physicians to differentiate between bacterial and viral infections
GSK and Sanofi initiate phase 1/2 clinical trial of COVID-19 adjuvanted recombinant protein-based vaccine candidate
Sanofi and GSK are scaling up the manufacturing of the antigen and adjuvant with the target of producing up to one billion doses in 2021
Using peptide-based T cell activation assays to identify COVID-19 epitopes
Read this application note to discover how T cell activation assays can help accelerate COVID-19 vaccine development