News & Articles
Data shows Abbott's FreeStyle Libre system dramatically decreases diabetic ketoacidosis-related hospitalizations
Additional real-world data demonstrate meaningful outcomes for people living with diabetes who use Abbott's glucose sensing technology
COVID-19 breakthrough: Dexamethasone reduces deaths in hospitalized COVID-19 patients
The RECOVERY trial has shown that the cheap and widely available steroid cut deaths by one-third among patients critically ill with COVID-19
Lonza and CELLINK join forces to offer complete 3D cell culture workflows
Lonza has collaborated with CELLINK to offer a comprehensive 3-dimensional (3D) bioprinting solution designed to advance complete 3D cell culture workflows
Menarini Silicon Biosystems begins distribution of rapid COVID-19 antibody test in North America
The test detects antibodies made by the immune system in response to infection by the novel coronavirus in as little as 10 minutes
GenNext Technologies and NeoProteomics announce an alliance in biomolecular footprinting
Companies team-up to promote the use of protein footprinting technologies, products, and contract research services in biopharmaceutical research and development
How PerkinElmer is helping to combat COVID-19
It's comprehensive SARS-COV-2 offerings span RT-PCR, high throughput RNA extraction, automation, ELISA and lateral flow based serology testing.
Analytik Jena celebrates 30th anniversary
30 stories from customers, partners and employees celebrate the past and look ahead to joint success in the future
Imperial to begin first human trials of new COVID-19 vaccine
Clinical researchers are this week set to begin human trials of a new coronavirus vaccine developed by researchers at Imperial College London
Cobra Biologics signs supply agreement with AstraZeneca for manufacture of COVID-19 vaccine candidate
The production agreement is part of AstraZeneca’s recently announced in-licensed programme with the University of Oxford
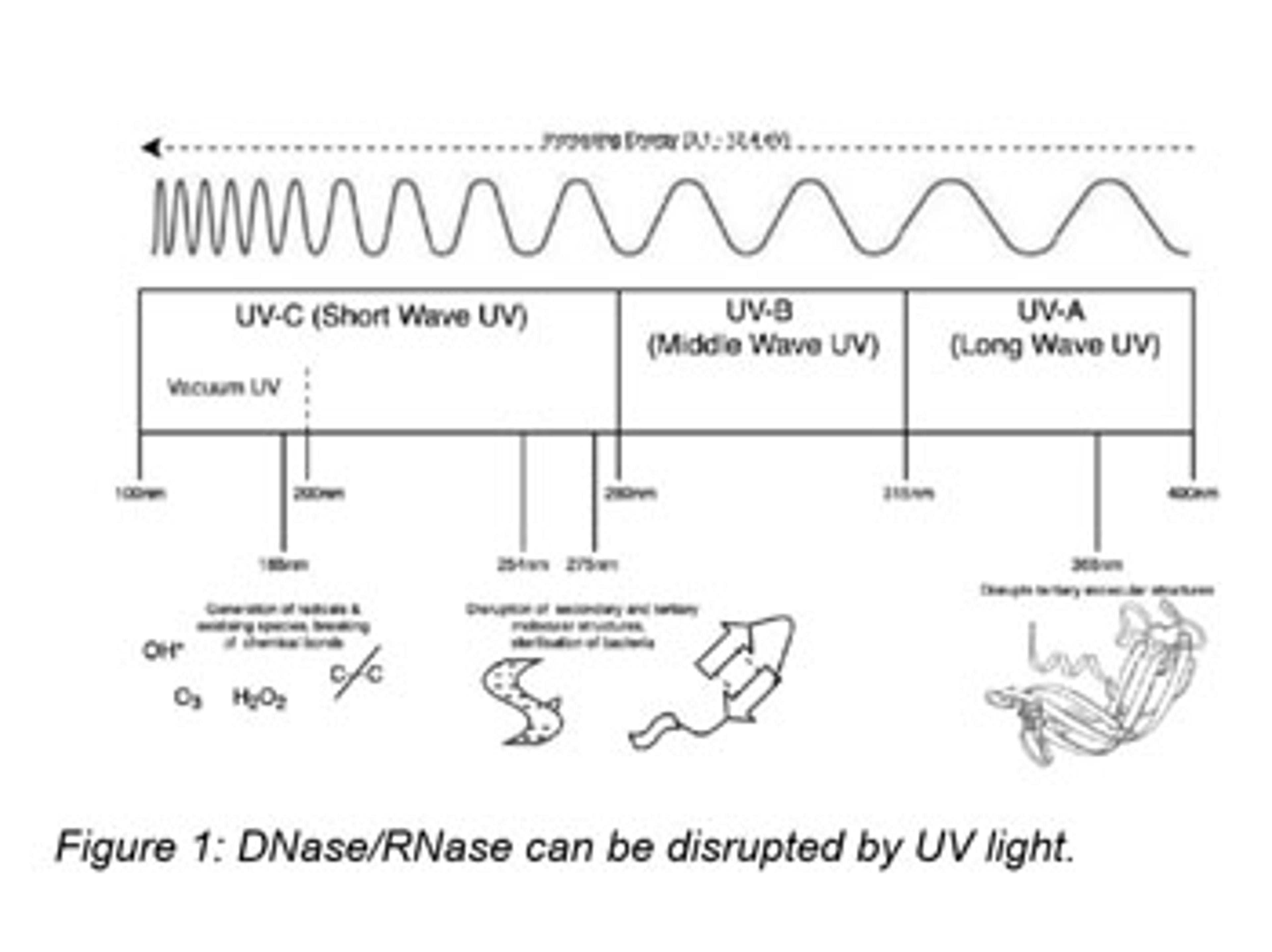
Are point-of-use filters really necessary for nuclease removal?
Undetectable levels of DNase and RNase in purified water
Promega announces CE marking for OncoMate MSI Dx Analysis System
New diagnostic test for MSI in solid tumors can inform physicians on immuno-oncology therapies and can guide treatment decisions for patients with Lynch-associated cancers
AstraZeneca to supply Europe with up to 400 million doses of Oxford University’s COVID-19 vaccine
Company exploring further additional global capacity to provide broad and equitable access
EKF opens for live chats at ENDO Online 2020
Diagnostics for glycemic monitoring and ketosis testing on show
New ASCO studies offer evidence for use of circulating tumor cells as a prognostic biomarker for advanced prostate and breast cancer
Results from prospective trials show a “clear prognostic value” for CTC counts
Next-generation analytical software strengthens data exploration
Thermo Scientific software suite expands capabilities in sample analysis with enhanced flexibility, reliability and customization
Fluxion Biosciences joins BloodPAC consortium to accelerate liquid biopsy development
Participation will help advance liquid biopsy adoption by collaborating among key industry stakeholders
Autoscribe Informatics expands African distributor network
Sciencescope becomes Autoscribe's Sub-Saharan Africa distributor
FDA authorizes first COVID-19 diagnostic test utilizing next generation sequence technology
llumina's COVIDSeq aims to generate information about the genomic sequence of the virus