News & Articles
Mammoth Biosciences partners with GSK on handheld CRISPR-based COVID-19 test
Driven by the gap in COVID-19 testing, the collaboration aims to create a fully disposable, rapid and handheld test for consumers
Indica Labs, Octo and Axle work with NIH to launch a global COVID-19 digital pathology repository
COVID-DPR is a virtual collection of high-resolution microscopic COVID-related human tissue images hosted at the National Institutes of Health
Rentschler Biopharma licenses Horizon Discovery’s CHOSOURCE platform
Platform to complement Rentschler’s cell line development offering for difficult-to-express proteins
Zymo Research granted emergency FDA authorization for quick SARS-CoV-2 RRT-PCR kit
A qualitative test for the detection of nucleic acid from SARS-CoV-2
AsiDNA™ to reverse cancer resistance to PARPi
Onxeo to present new preclinical data at AACR 2020 illustrating the ability of AsiDNA™
University of Paris researchers utilize Fluidigm mass cytometry for COVID-19 patients
Study suggests that type I IFN deficiency in the blood could help define a high-risk population
Pfizer, BioNTech dose first US participants with COVID-19 vaccine
The COVID-19 vaccine trial is part of a global development program that will uncover the safety and immunogenicity of four mRNA vaccine candidates.
Agilent PD-L1 assay receives FDA approval for use as a companion diagnostic
Assay will help guide treatment decisions in cases of non-small cell lung cancer
Developing a 10-minute fingerprint test for COVID-19
A portable and non-invasive, fingerprint-based method, ideal for on-site testing in care homes and the workplace
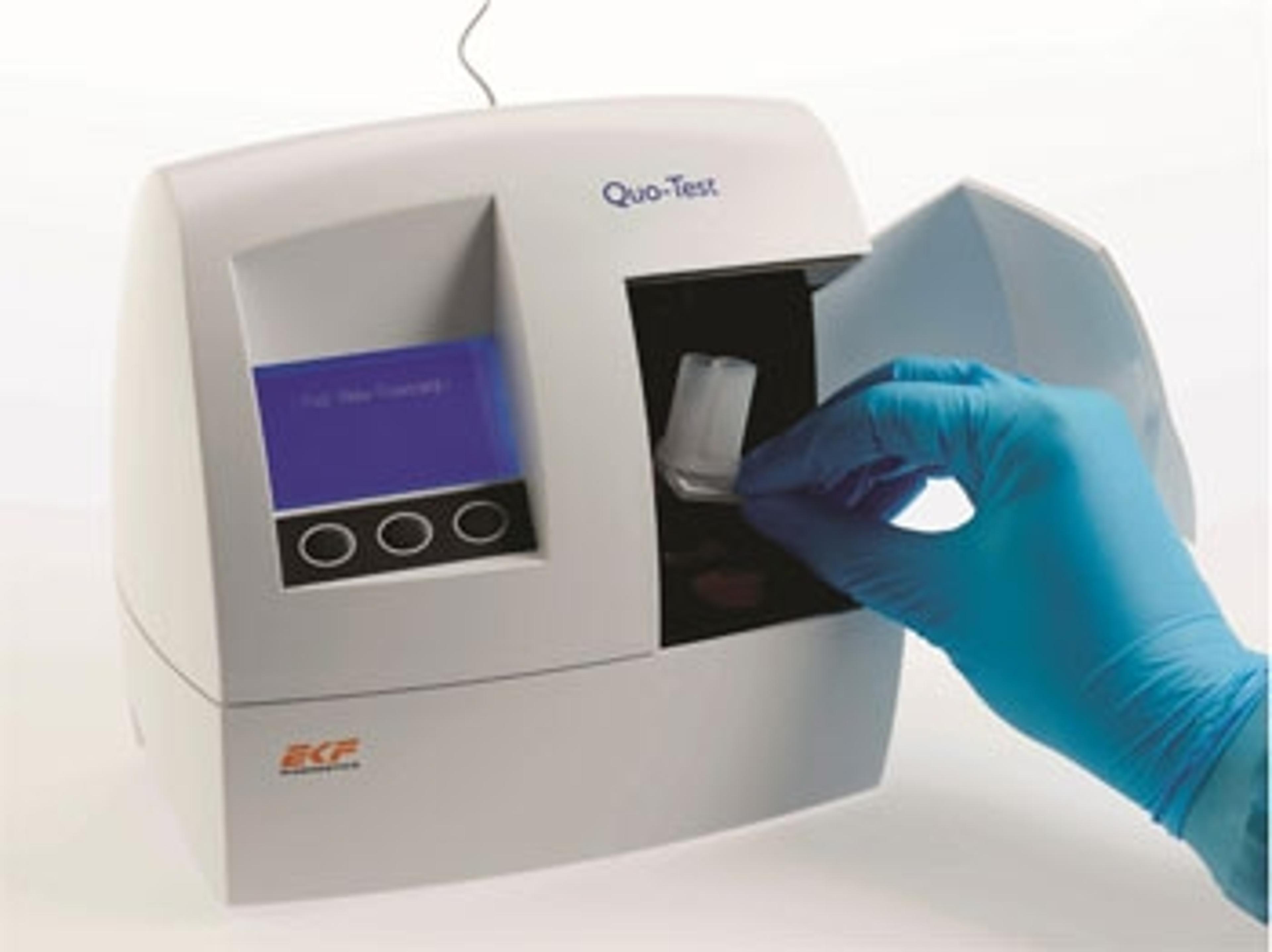
Distribution agreement expands HbA1c POC testing reach in Middle East & Africa
Leading HPLC analyzer manufacturer Tosoh Europe partners with EKF for Quo-Test HbA1c point-of-care analyzer
Renishaw ramps up production of ventilator components
Mass production comes as part of a nationwide effort to support the NHS in the fight against COVID-19
FDA authorizes first at-home COVID-19 diagnostic test using saliva specimens
The U.S. Food and Drug Administration has authorized the first coronavirus test using at-home collection of saliva samples
Ortho Clinical Diagnostics partners with Quest in the fight against coronavirus
Partnership to broaden the availability of COVID-19 antibody testing with 100% specificity
Cambridge researchers design an open-source ventilator for low-income countries
A research team at the University of Cambridge has designed a ventilator for treating COVID-19 patients that can be quickly and cheaply manufactured
Thermo Fisher Scientific announces SARS-CoV-2 GlobalAccess Sequencing Program
Thermo Fisher Scientific will provide 50 Ion Torrent Genexus Systems for consortia members to support global COVID-19 research
RedHill Biopharma receives FDA approval for COVID-19 clinical study with Opaganib
The randomized, double-blind, placebo-controlled study aims to enroll up to 40 patients with moderate-to-severe COVID-19 pneumonia in the U.S
PerkinElmer receives EUA for COVID-19 antibodies test
Clinical laboratories certified to perform high complexity tests under Clinical Laboratory Improvement Amendments (CLIA) can immediately begin using this ELISA