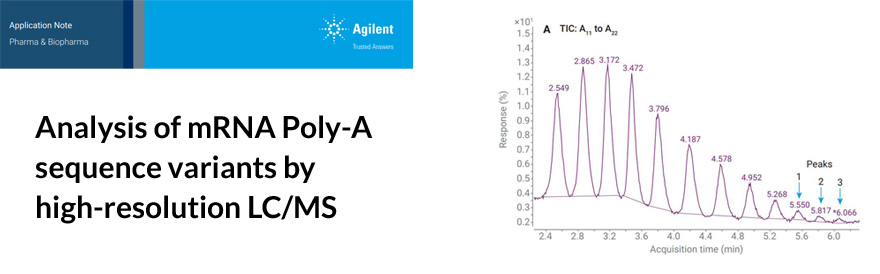
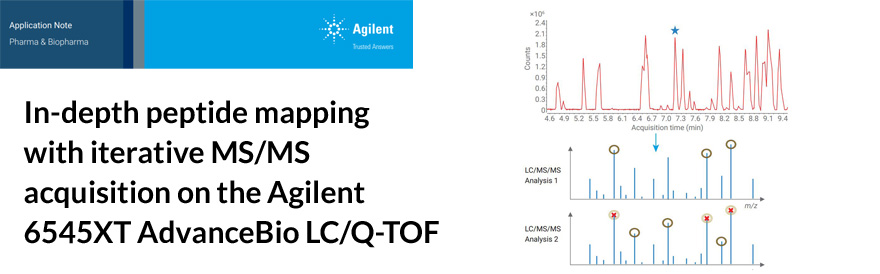
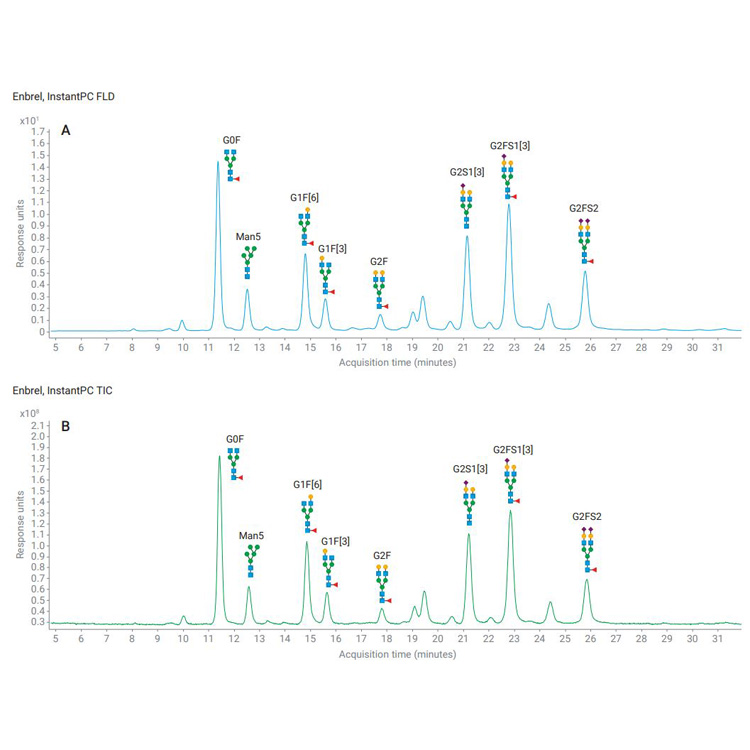
Biopharmaceuticals have revolutionized the treatment of a number of
diseases. With this success, and continued advances in biotherapeutic options, consistently reliable
manufacturing and quality control processes are required. Accurate and robust analytical testing equipment
and methodologies are critical.
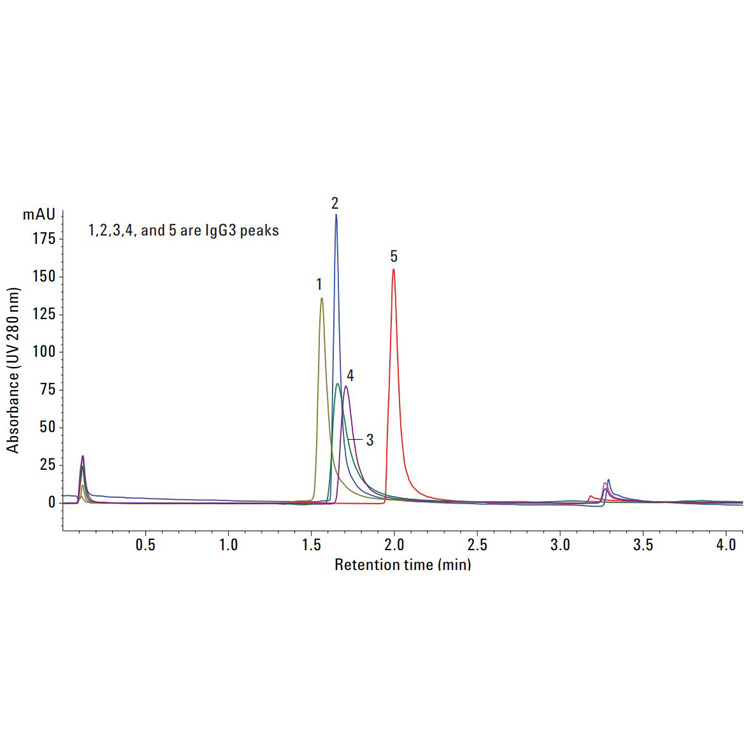
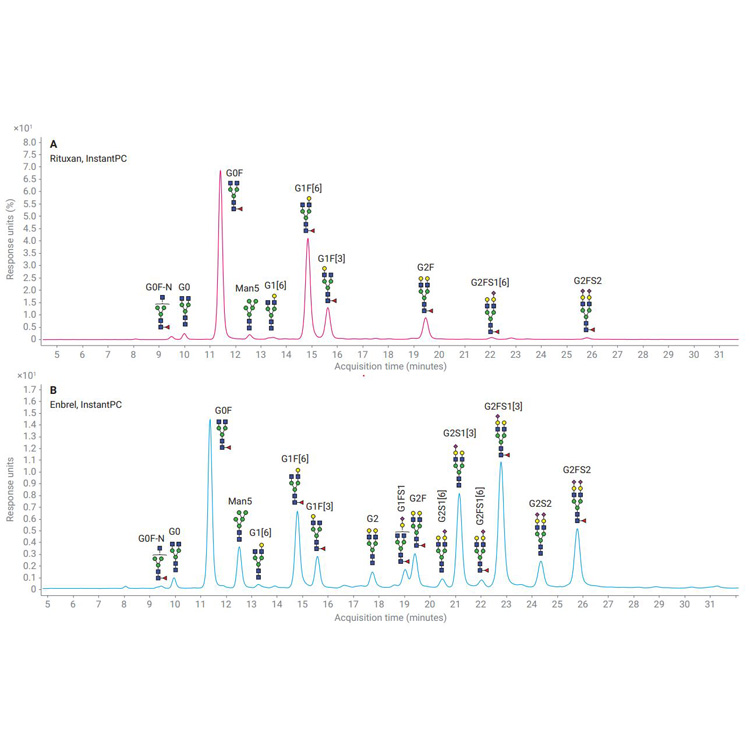
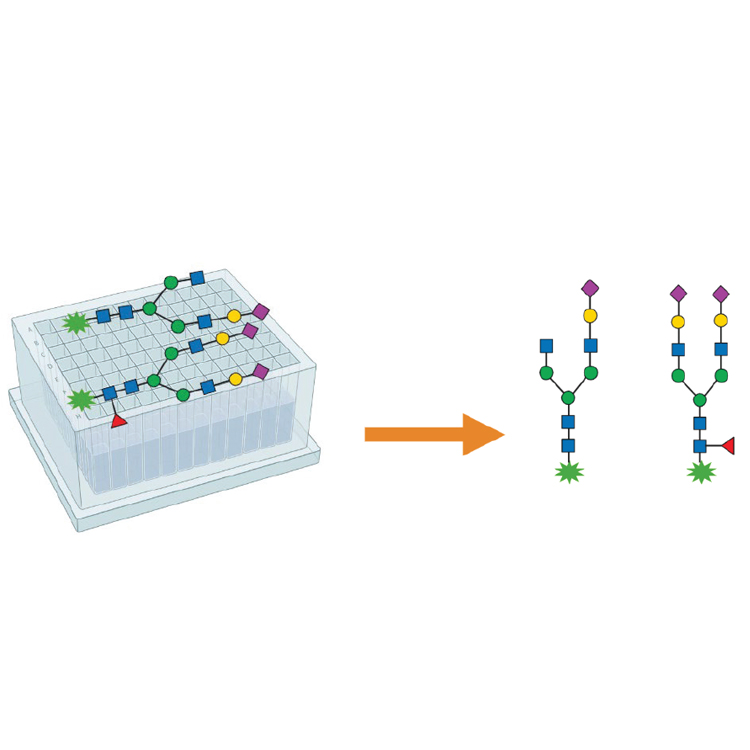
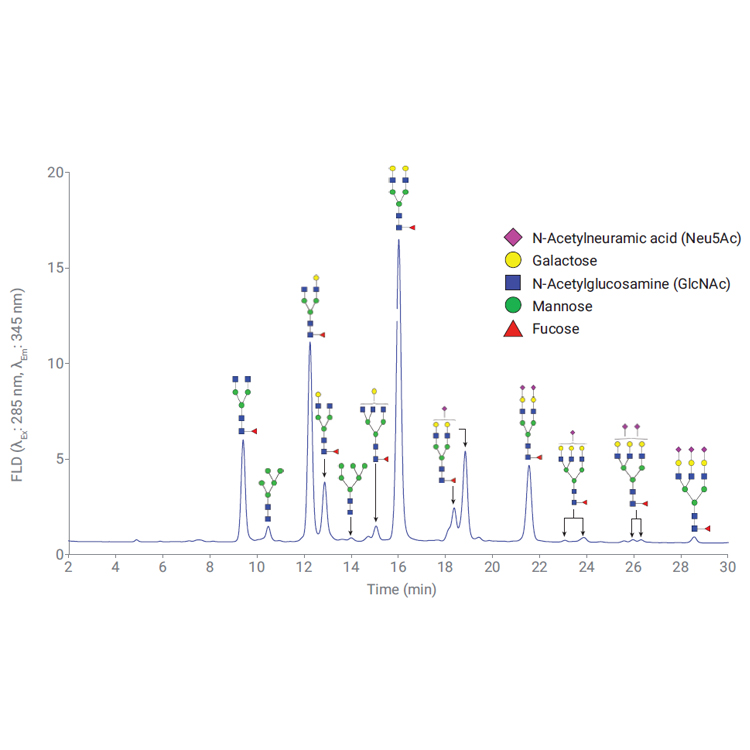
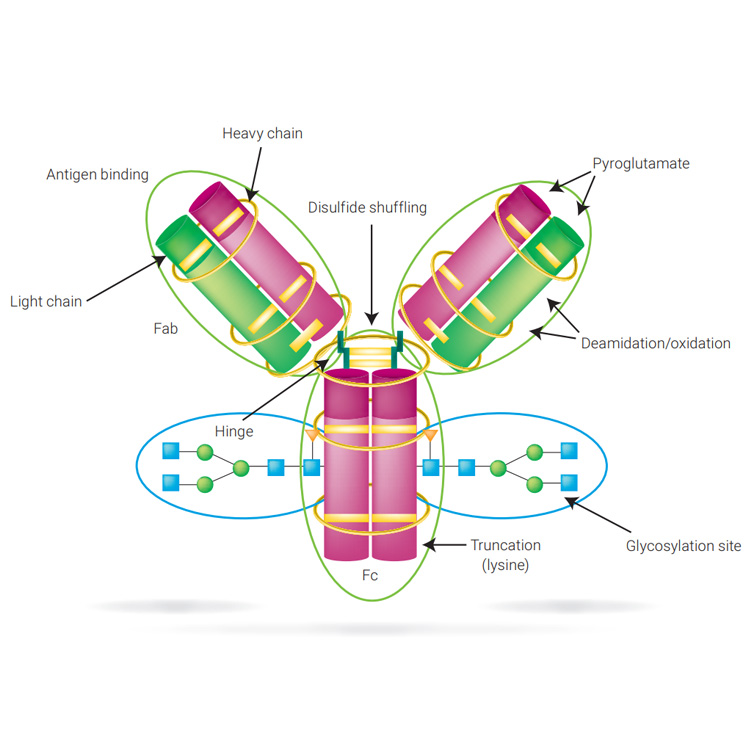
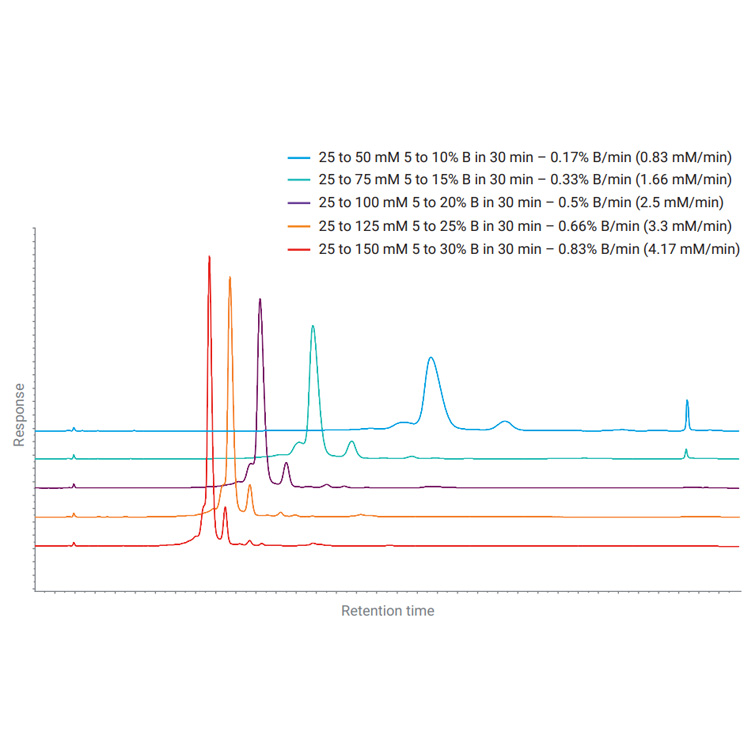
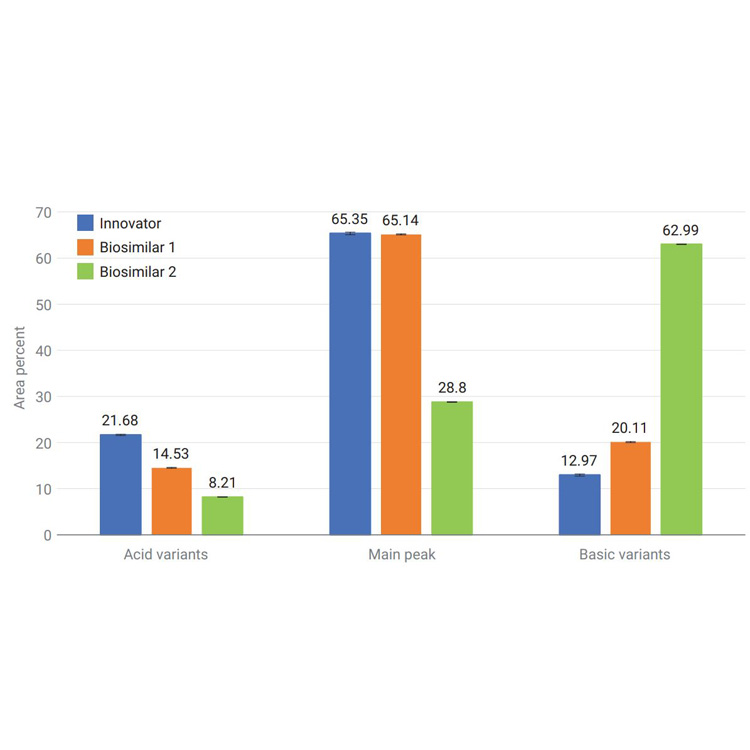
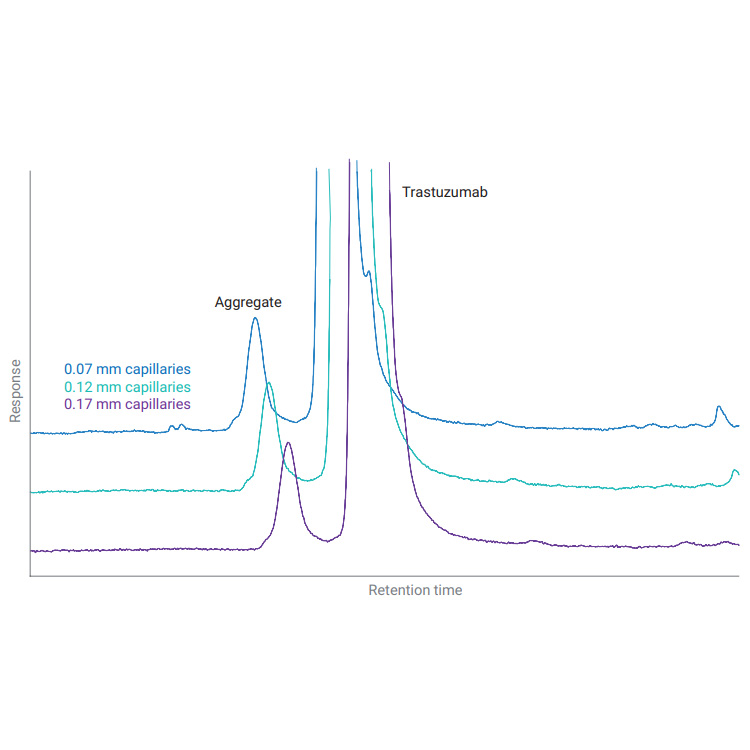
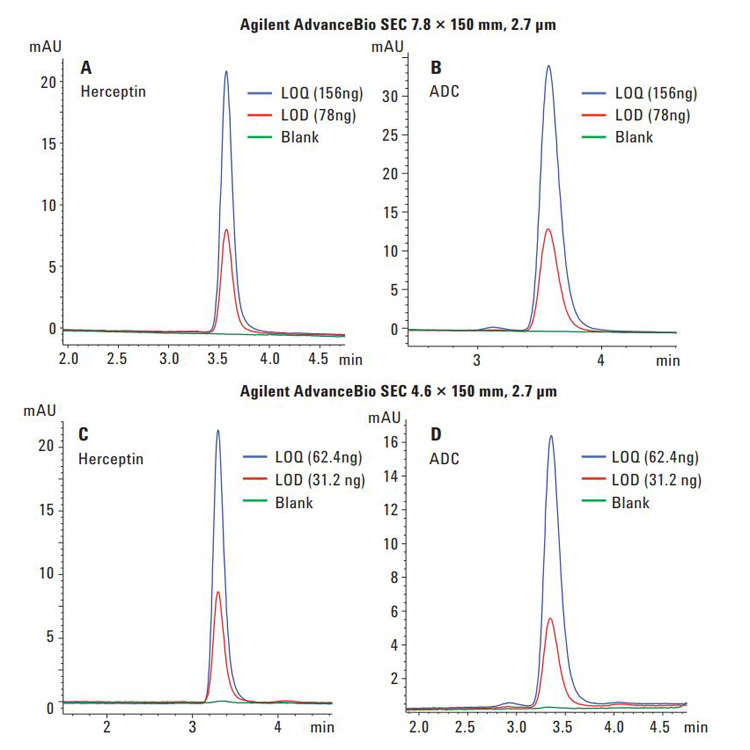
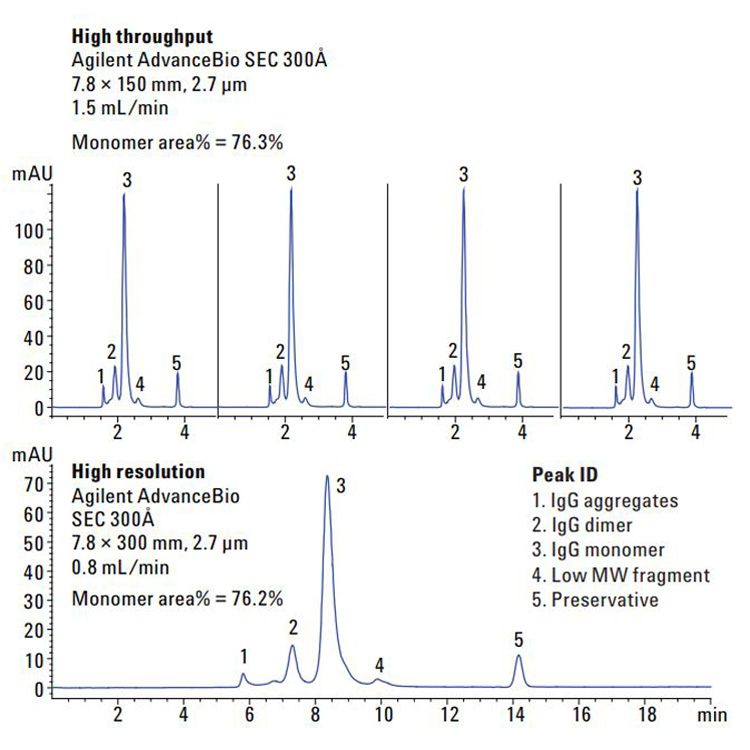
Biotherapeutics such as monoclonal antibodies (mAbs) are typically produced using recombinant methodologies and bioprocessing technology, which can result in the generation of impurities, post-translational modifications (PTMs), and protein aggregates that can affect drug safety and efficacy. Identifying and analyzing critical quality attributes (CQAs) of complex biotherapeutic molecules is therefore a fundamental process throughout each development stage of biopharmaceutical protein production.
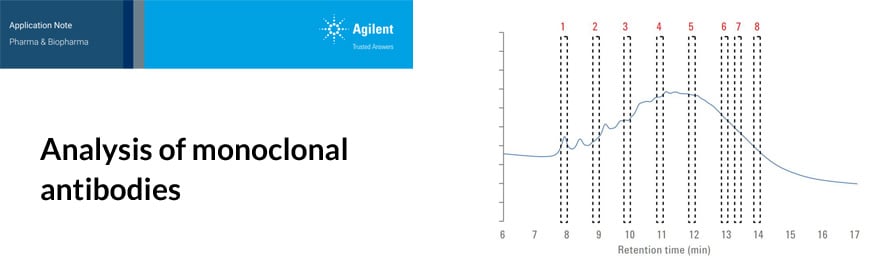
In this resource, explore some of the chromatographic applications for CQA monitoring within the most common bioanalysis workflows. See how Agilent Biopharma and AdvanceBio HPLC columns and technology can help ensure reliable analytical results and effective biotherapeutics.