Diasorin Ltd
The products developed and distributed by the Diasorin Group are designed specifically for clinical diagnostics applications. They are used by diagnostic laboratories that are part of hospital facilities or operate independently (private diagnostic services laboratories). For the most part, they help physicians in diagnosing different diseases (diagnostic benefit), determining the progress of a disease (prognostic benefit) and assessing the effectiveness of a drug treatment (monitoring benefit).
The market for in vitro diagnostics comprises the following product categories, each of which requires different support technologies:
infectious Immunology;
immunochemistry;
clinical chemistry;
hematology, Histology, Cytology;
microbiology;
genetic Testing.
Within the in vitro diagnostics segment, the Diasorin Group specializes in developing, producing and distributing immunodiagnostics products, which comprises the immunochemistry and infectious immunology product categories.
LIAISON® XL
LIAISON® XL is a fully automated chemiluminescence analyzer, performing complete sample processing (sample pre-dilutions, sample and reagent dispensing, incubations, wash processes, etc.) as well as m...
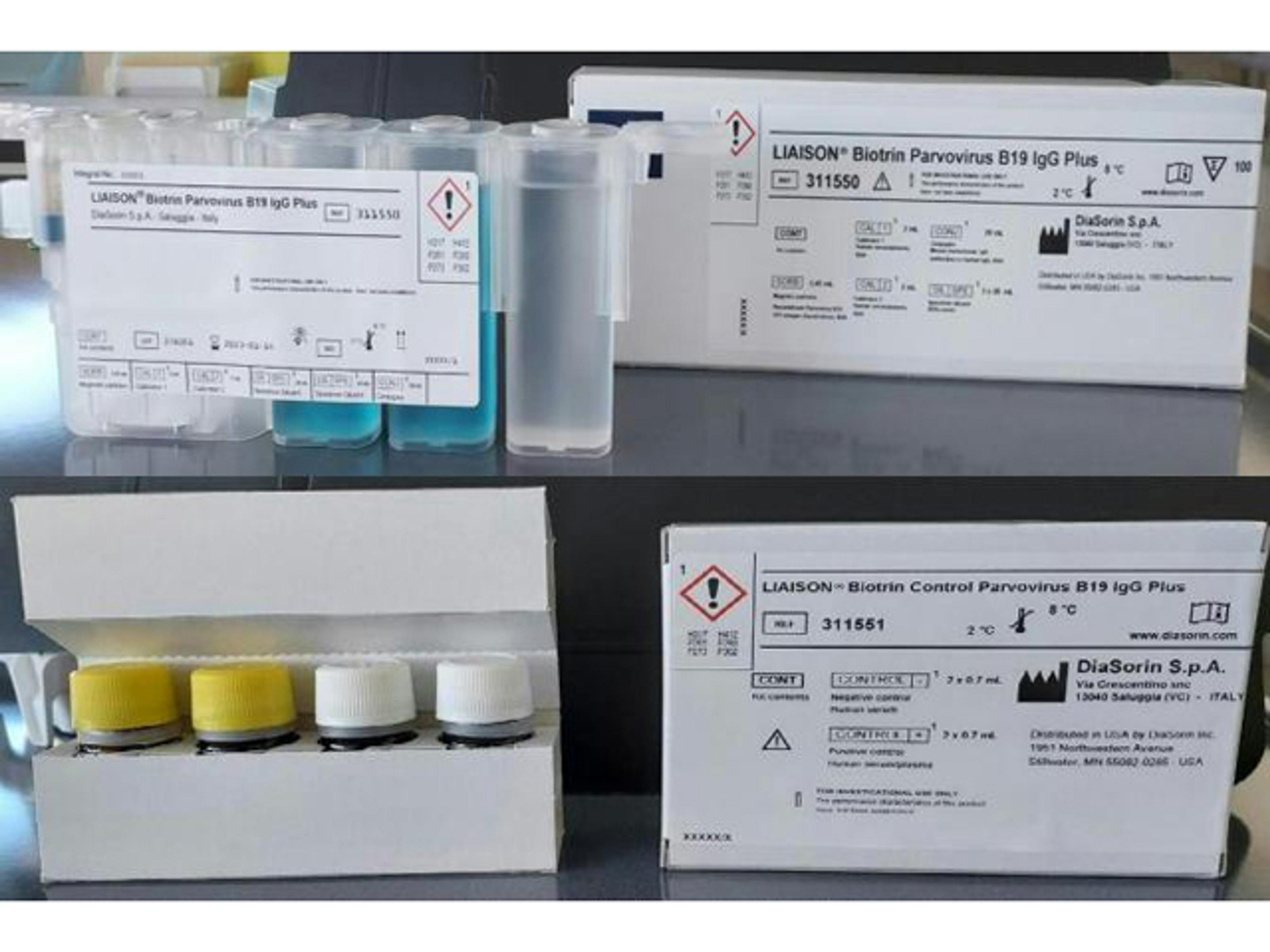
LIAISON® Biotrin Parvovirus B19 IgG Plus
LIAISON® Parvovirus B19 IgG is a CLIA assay for the quantitative determination of specific IgG antibodies to Human Parvovirus B19 in human serum or plasma samples.
LIAISON® SARS-CoV-2 TrimericS IgG
The LIAISON® SARS-CoV-2 TrimericS IgG assay enables the reliable detection of Trimeric S spike protein IgG antibodies – the body’s natural defense response against SARS-CoV-2.
The products developed and distributed by the Diasorin Group are designed specifically for clinical diagnostics applications. They are used by diagnostic laboratories that are part of hospital facilities or operate independently (private diagnostic services laboratories). For the most part, they help physicians in diagnosing different diseases (diagnostic benefit), determining the progress of a disease (prognostic benefit) and assessing the effectiveness of a drug treatment (monitoring benefit).
The market for in vitro diagnostics comprises the following product categories, each of which requires different support technologies:
infectious Immunology;
immunochemistry;
clinical chemistry;
hematology, Histology, Cytology;
microbiology;
genetic Testing.
Within the in vitro diagnostics segment, the Diasorin Group specializes in developing, producing and distributing immunodiagnostics products, which comprises the immunochemistry and infectious immunology product categories.