Troubleshooting
Quality control
Good versus bad dPCR data
There are several dPCR instrument options on the market, but not all systems produce the same quality of results. Learn how to distinguish high-quality from low-quality dPCR data, explore potential causes underlying specific data issues, and discover the difference between Droplet Digital PCR and other Digital PCR Systems.
 Download
Download
Achieve accuracy and precision
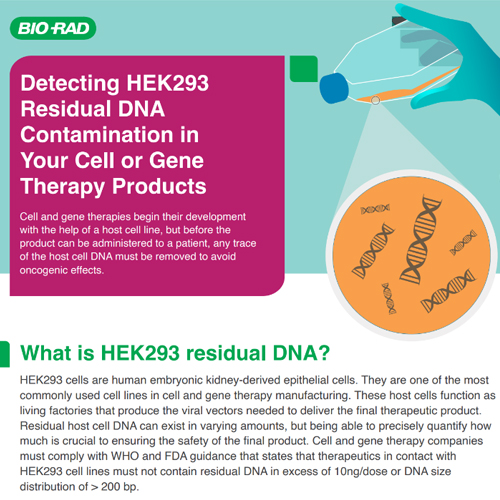
Whether evaluating transgene copy number or verifying the absence of microbial
pathogens, discover how Bio-Rad's comprehensive range of GMP-ready ddPCR assays can effectively ensure
the safety of your cell and gene therapy manufacturing process.
 Download
Download