How to Buy Gas Chromatography Technology
1 Sept 2024
Gas chromatography (GC) is an analytical separating technique used to separate volatile and semi-volatile substances from complex mixtures. The method consists of a gaseous mobile phase, usually an inert carrier gas, such as helium, and a liquid stationary phase adsorbed onto an inert cylindrical solid support called a column. In this fourth edition eBook, we present a guide to all the key considerations when purchasing the best GC columns to meet your lab’s needs.

The optimization of chromatographic methodology starts with the column selection. In this guide, discover the latest advice on how to choose the best GC column for your experiments. Based on four significant factors, column selection is different for every experiment.
Contents
1. Introduction
Gas chromatography is an analytical separating technique used to separate volatile and semi-volatile substances from complex mixtures. The method consists of a gaseous mobile phase, usually an inert carrier gas, such as helium, and a liquid stationary phase adsorbed onto an inert cylindrical solid support called a column.
Once the sample to be analyzed is injected into the GC system, an isothermal oven temperature or temperature gradient is applied to the column. The compounds in the mixture interact with the stationary phase. Depending on the type of interaction and the boiling points of the compounds, separation occurs.
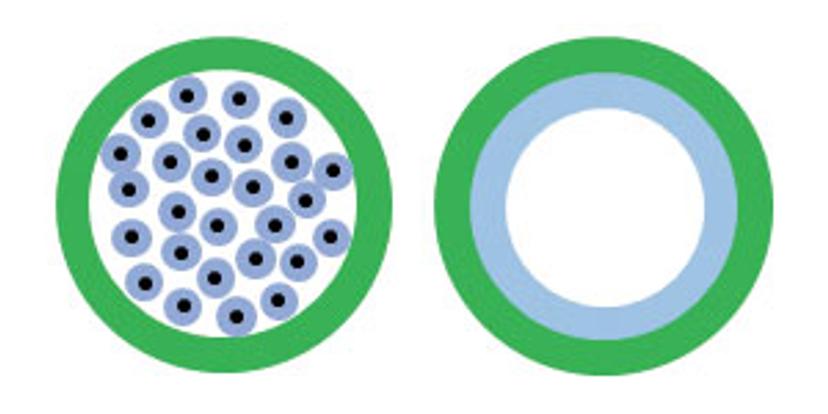
Figure 1: Packed GC columns (left) and capillary GC columns (right). Stationary phase appears in blue.
GC columns are broadly classified into two types: packed and capillary (figure 1). The packed columns are filled with inert solid material coated with the liquid stationary phase, while the capillary columns maintain a hollow interior with the stationary phase coated along its inner walls. Packed columns are preferred for analysis of gas samples, but for most analytical separations, capillary columns are more efficient and provide good peak separation and consistent results.
Compound separation in gas chromatography
The analytical separation of volatile and semivolatile substances from complex mixtures can be carried out using gas chromatography. In gas chromatography, separation of compounds is largely based on their boiling points, as well as intermolecular reactions. Whilst elution generally follows the boiling point of a compound, with a higher boiling point indicating a higher retention time in the column, intermolecular reactions between the solute and stationary phase also play a part in separation. As such, two analytes with similar boiling points can be separated on the basis that they have a different chemical structure.
What are the components of a GC capillary column?
There are three distinct layers that make up the cross-section of a capillary GC column (figure 2):
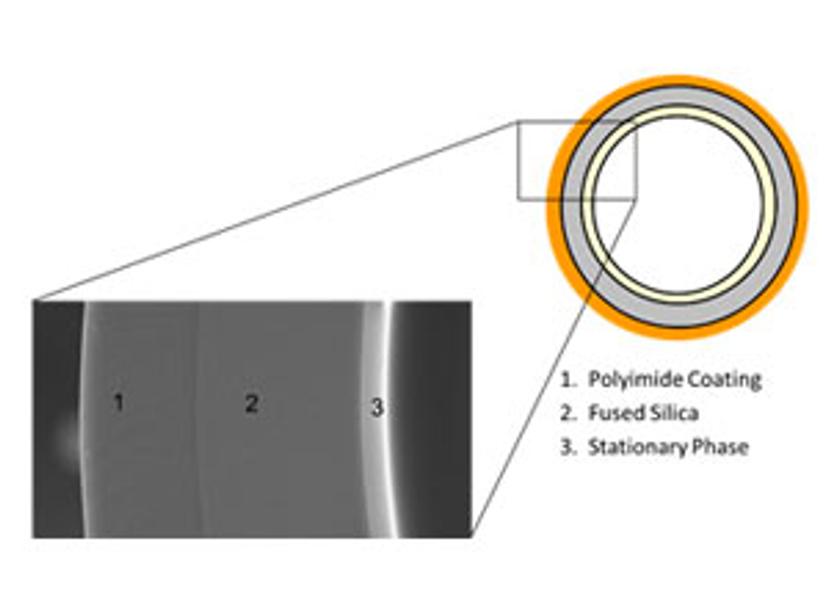
Figure 2: The cross-section of a capillary GC column. Image courtesy of Phenomenex.
1. Polyimide coating: This is a protective coating applied to the outer surface of the column. It not only gives the column its distinctive brownish appearance but also makes the column flexible and resistant to temperature.
2. Fused silica: The main material with which the column is built needs to be inert with the compounds being separated. Fused silica, which is synthetic quartz of high purity, has been a reliable and routinely used material for GC columns. It is supported with an outer layer of polyimide coating to add strength and an inner coating of stationary phase that performs the separation.
3. Stationary phase: A thin film of stationary phase coated on the inner wall of the capillary column serves as the most important factor in selecting a GC column. The fundamental rule in choosing the stationary phase for your application is ‘like dissolves like’. Analytes interact better with stationary phases of similar chemical nature, yielding a better separation.
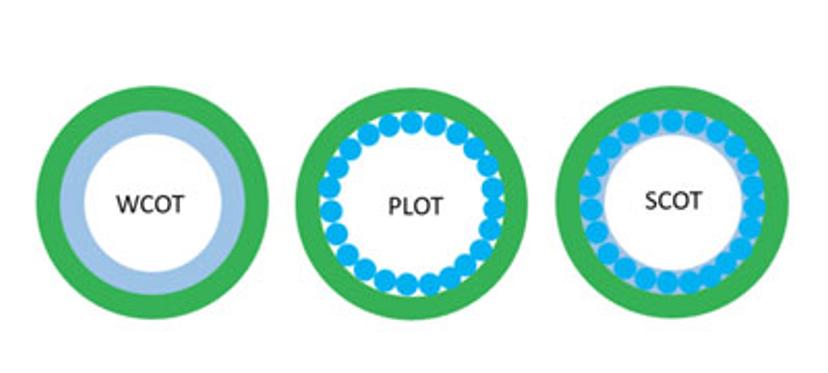
Figure 3: There are three categories of GC columns, depending on the structure of the stationary phase: (i) Wall-coated open tubular column (WCOT), (ii) Porous-layer open tubular column (PLOT), and (iii) Support-coated open tubular column (SCOT).
The structural characteristics of the stationary phase divide GC columns into three categories (figure 3): (i) Wall-coated open tubular column (WCOT), (ii) Porous-layer open tubular column (PLOT), and (iii) Supportcoated open tubular column (SCOT). A wall-coated open tubular column (WCOT) consists of a thin film coated on the inner wall. The porous-layer open tubular column (PLOT) is a porous solid layer on the capillary’s inner wall, while the supportcoated open tubular column (SCOT) includes a liquid stationary phase in addition to a porous support.
2. How to choose a capillary GC column
Once you have identified the analytes that you want to separate, there are a number of additional considerations in a GC column to ensure a high-quality separation and a clean chromatogram.
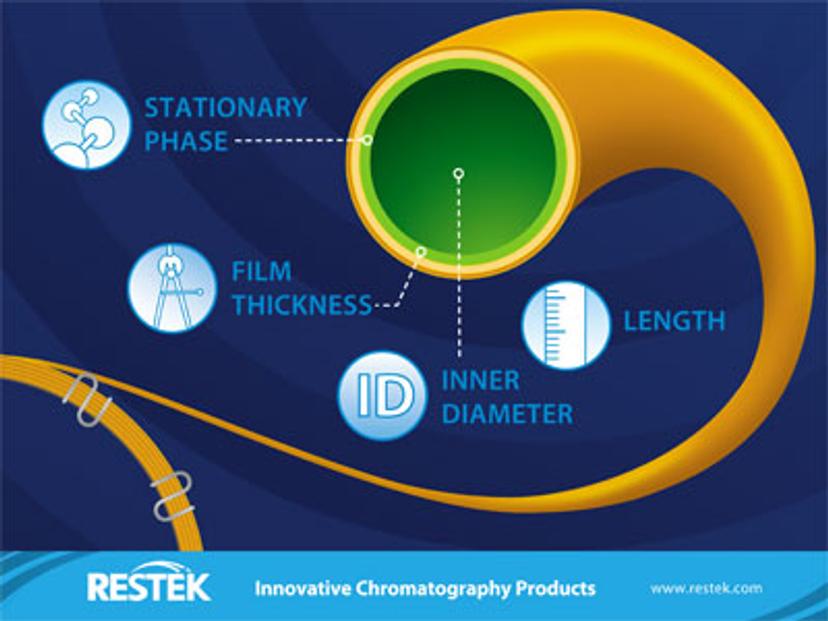
Figure 4: When choosing a column there are four factors you need to consider. Image provided courtesy of Restek.
Listed below are the factors to consider when choosing a GC column (figure 4):
- Stationary phase
- Column length
- Column internal diameter (I.D.)
- Film thickness
Selecting the stationary phase
The master resolution equation (figure 5) defines that the best resolution obtained for two solutes in a GC separation comprises three terms: efficiency, selectivity and retention. Of these, the biggest impact on resolution is made by selectivity which, in turn, directly relates to the stationary phase.
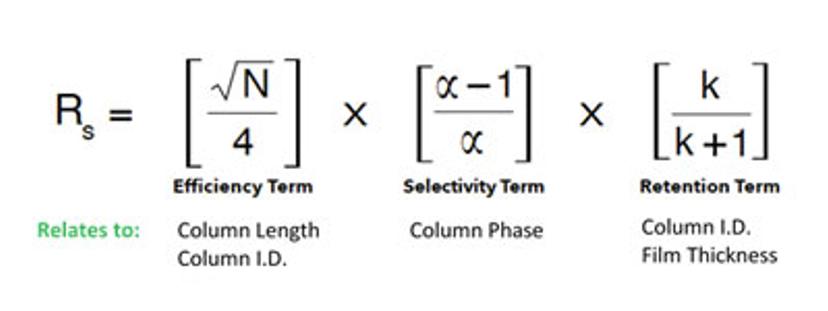
Figure 5: The master resolution question breaks down the relationship between column length, phase, I.D. and film thickness.
Your choice of a stationary phase in the GC column has the highest impact on the separation, and eventually, your data. Following the principle of ‘like dissolves like,’ the interaction between the analytes of interest with the stationary phase forms the core of chromatographic separations. The physical and chemical properties of the analyte and the stationary phase govern the analyte-phase interactions. In separating two compounds, if the interaction between one analyte and the stationary phase is stronger, it is retained longer in the column, and helps achieve a good separation. Alternatively, with certain stationary phases, when two analytes do not separate (i.e. they coelute), changing the stationary phase by altering its chemistry can help achieve a separation. GC column manufacturers provide a wide range of capillary column phases to choose from, with each phase providing a specific chemical property.
The first step in selecting a stationary phase is to review the large volume of scientific publications in your area of application. Chances are that you will find a stationary phase well suited for your experiment. Additionally, GC column manufacturers provide phase selection charts, compiled from the numerous GC successes in different industries and applications, that can aid your selection.
If, however, your application is unique and has no reference, it is important to know the physical and chemical properties of the analyte of interest. Listed below are some factors that can help with your method development.
A stationary phase is selected on the following criteria:
- Phase selectivity
- Phase polarity
Phase selectivity is the ability of the stationary phase to differentiate between two analytes by identifying the differences in their chemical or physical properties. It is directly related to the composition of the stationary phase, and how it reacts with the targeted compounds’ intermolecular forces.
Phase polarity is determined by the structure of the stationary phase. The polarity of the substituted groups in the stationary phase and their abundance affects the overall polarity of the column. Phase polarity is often mistaken to be the most influential factor in solute resolution, which, in fact, is phase selectivity. Polarity is, however, one of the many factors that affect retention and, therefore, peak separation. Phase polarity follows the ‘like dissolves like’ principle. Non-polar compounds are better retained and separated by non-polar columns, and vice versa.
Selecting column length
The efficiency of a column is directly proportional to the column length. Longer columns, therefore, are meant to yield a higher resolution. However, column resolution is related to the square root of the column length. This means increasing the length will only have a limited improvement in terms of resolution.
Column length is directly related to run time. Increasing the column length will, therefore, also increase the time taken for analyte separation (table 1).

Table 1: The relationship between the length of the column, resolution and run time.
In summary, longer columns yield a greater resolution but increase back pressure and run times, while shorter columns are preferred for applications where resolution is not the criteria, for example, when screening for analytes. In general, the column I.D. is often changed along with column length to yield the desired separation (figure 6).
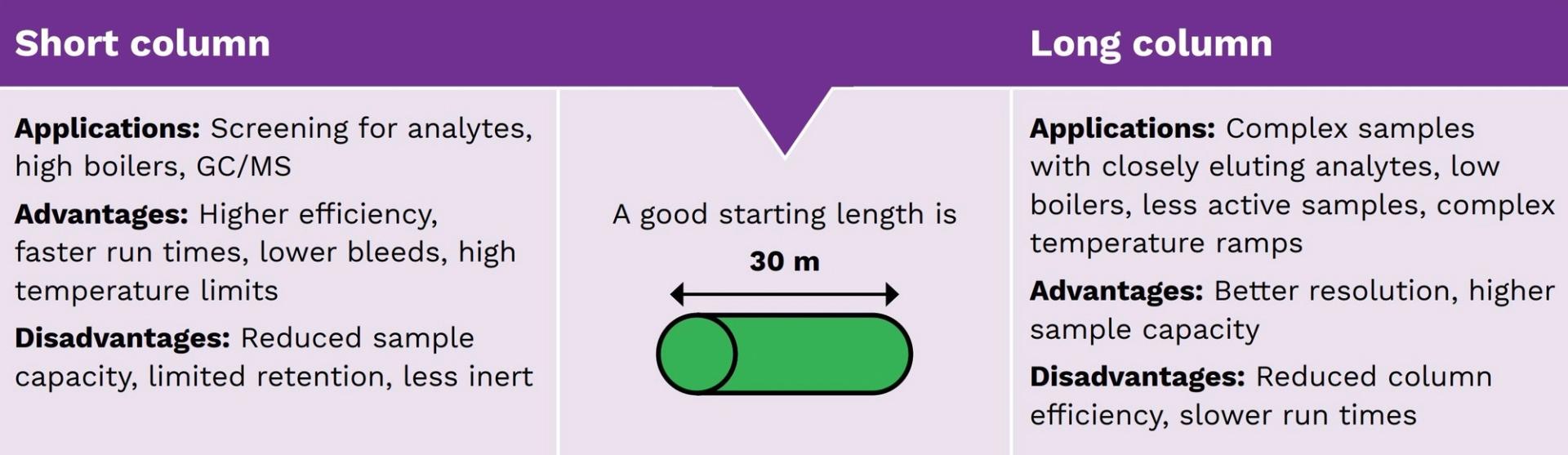
Figure 6: Considering the length of a GC column.
Selecting column internal diameter
The internal diameter (I.D.) of a column directly affects the column efficiency (i.e. the number of theoretical plates) and the sample capacity (i.e. the amount of sample that can be injected into the column without causing an overload). The column efficiency, measured in plates (N), is inversely proportional to column I.D.: it increases as column I.D. decreases. Whereas the sample capacity is directly proportional to column I.D.: it decreases as the column I.D. decreases (figure 7).
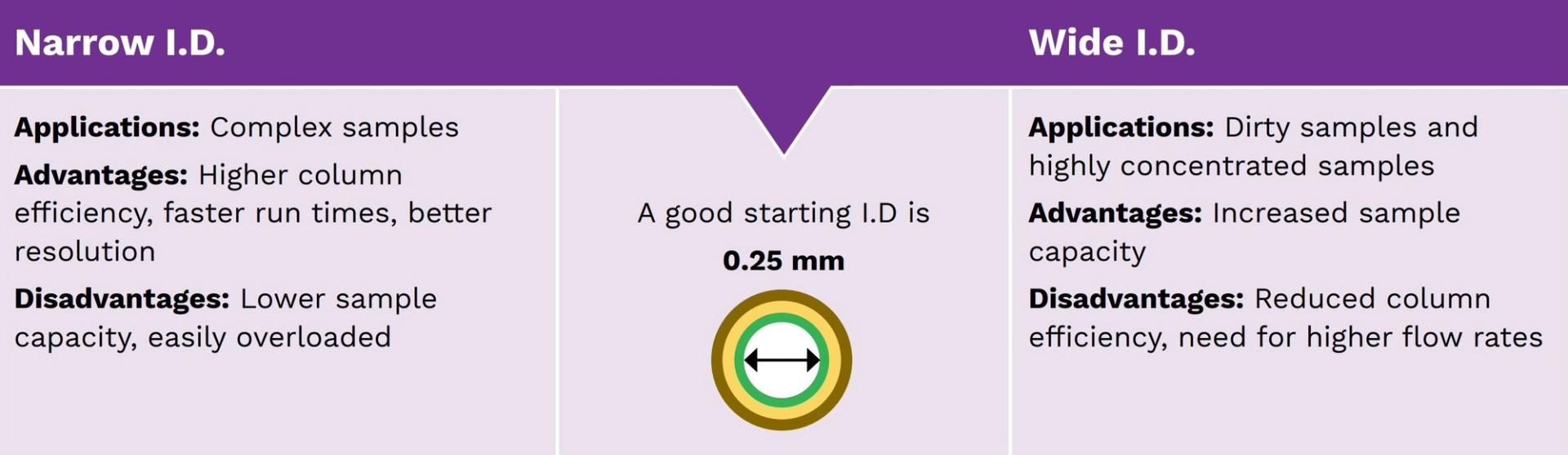
Figure 7: Considering the internal diameter of a GC column.
Selecting film thickness
The thickness of the stationary phase on the inner wall of the capillary column determines solute resolution and sample capacity. The thinner the film coating, the higher the resolution, but lower the sample capacity — and vice versa. Decreasing the film thickness yields sharper peaks, reduces column bleeds and increases signal-to-noise ratio. Whereas increasing the film thickness increases sample capacity and reduces maximum operating temperatures, but decreases analyte-phase interaction (figure 8).
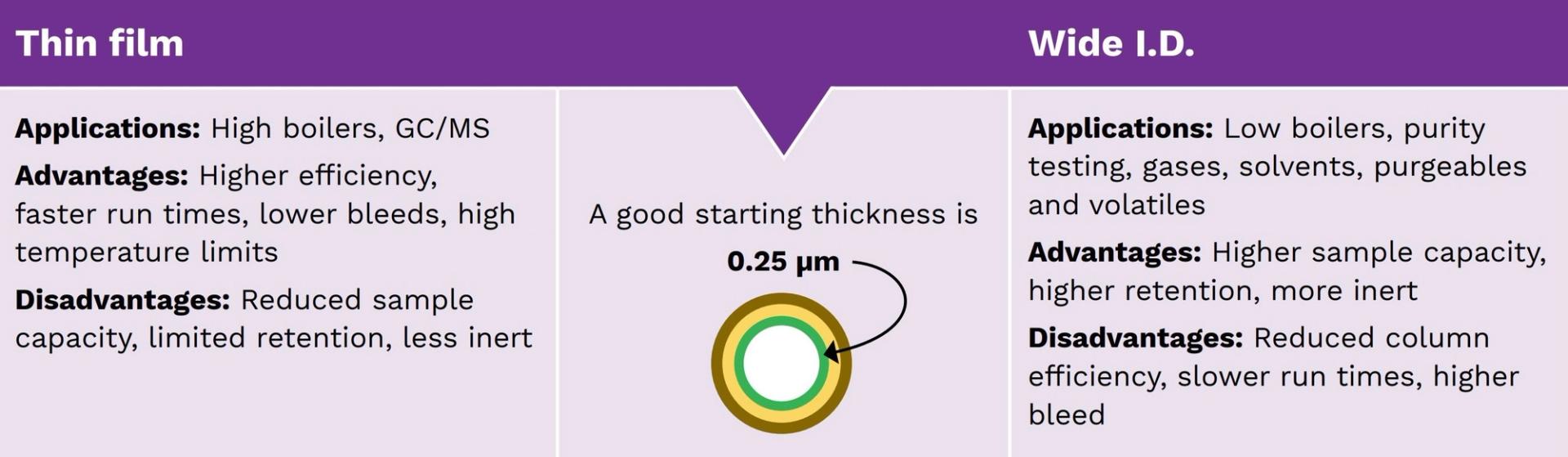
Figure 8: Considering the film thickness of a GC column.
3. Additional factors to consider
GC Accelerator Kits
If you need to speed up your sample analysis, consider purchasing a GC Accelerator Kit. These kits work by reducing the volume of the oven, allowing for faster ramp rates to be attained – reducing the oven cycle time and enabling increased sample throughput.
One such kit, from Restek, is designed specifically for Agilent’s 6890 and 7890 instruments. You can simply apply an existing method, scaled down using Restek’s EZGC Method Translator, allowing you to obtain the same chromatographic separation in a fraction of the time.
Column contamination
A contaminated column is one of the most common causes of performance degradation in GC systems. The contaminants typically originate from injected samples. Over time, semi-volatile and nonvolatile sample components, upon injection, accumulate in the injector and on the column surface. The semi-volatile contaminants accumulate in the column, but eventually elute out, however, the non-volatile ones do not elute and continue accumulating. Loss of peak size and shape, and peak tailing, are usual symptoms of a contaminated column. Additionally, the stubborn non-volatile residues can reflect on the baseline of the chromatogram, causing instability, wander, drift and ghost peaks.
To tackle column contamination, consider heating the column to a high temperature, known as ‘baking out’ at the end of your run. This helps remove high-boiling contaminants from the columns. Care should be taken to control the temperature and time of such a bake-out so as to prevent thermal damage. Alternatively, in some GC columns, soluble contaminants can be removed with solvent rinsing, starting with the most polar solvent first. It is advisable to check whether your column’s stationary phase is compatible with a solvent rinse before proceeding as it can cause swelling of the stationary phase.
Finally, consider cutting off contaminated parts of the column, usually 1-2 loops from the column’s inlet, to retain the remaining functional parts of the column, as featured in this video from Restek. Note that each GC column is different. Please read the manufacturer’s instructions carefully before proceeding towards troubleshooting.
GC column manufacturers offer application specific searches to aid your selection of GC columns,
Guard columns and retention gap
The use of guard columns, especially when samples injected are dirty, can increase the life of the analytical column. A short, deactivated fused silica tubing (0.5 – 1 meter) installed between the injection port and the analytical column serves as the guard column. It catches contaminant residues injected with the sample and prevents it from accumulating on the analytical column. The guard column, when longer (between 3 – 10 meters in length), can serve as a retention gap, which allows for an improvement in peak shapes for early eluting analytes, stationary phase and GC conditions.
In an interview with a technical specialist at Phenomenex, this article highlights the most common issues with GC and a recommended troubleshooting process for researchers.
Always buy from a reliable source
When investing in GC columns, it’s important to choose a reliable source. Manufacturers perform multiple tests on the column before it finds its way to the customer. These tests include resolution of critical pairs, bleed test, retention factor of probe analytes, efficiency of the column and so on. Choose a reliable source that performs testing on individual columns instead of batch testing. To aid your decision, you can find out what other scientists think of their GC columns by reading their reviews under GC products in the SelectScience® product directory.
Application search programs
GC column manufacturers offer application-specific searches to aid your selection of GC columns, as well as the relevant system considerations and experimental conditions. Listed below are a few application search programs:
- Restek Technical Library
- Phenomenex Application Search
- Agilent Application Finder
- Shimadzu Industries Library
…among others.
Top tips for choosing a GC column
Expert advice from Dr. Mark Badger, Restek:
- Be sure to read the literature and see what other analysts have used or recommend for matching the column phase to the application. The applications resource from Restek is a great way to simplify your search.
- Make sure the column that is chosen is compatible with the type of detection being used.
- Consult with a technical specialist if you have questions.
- Model your analysis on a method development tool like ProEZGC to optimize your method before you buy a column.
4. Top GC applications
Cannabis analysis
Changing cannabis legislation for both medicinal and recreational purposes has had a major impact on the ways cannabis is processed, from the rise in CBD use to quality testing. Gas chromatography has been a mainstay for many labs. In the application note below (figure 9), discover a wealth of information on the analytical options available for cannabis testing, from column choice to novel methodologies that tackle the toughest of analytical problems.

Figure 9: This cannabis application compendium highlights the importance of this application area.
Also, watch the award-winning video from Dr. Nathaly Reyes Garcés, LC application scientist at Restek, as she explains a simple workflow for the accurate analysis of pesticides in cannabis products via solvent extraction and GC analysis.
Semi-volatile detection
Volatile organic compounds (VOCs), are a global concern and be found in samples ranging from wastewater, groundwater and soil. Semi-volatiles contaminants are a broad range of compounds including hydrocarbons, nitroaromatics, PAHs and esters. These compounds can be found in a wide range of sources including pesticides and herbicides, solvents, flame retardants and cleaning agents.
Due to the varying composition of these compounds, it is important to have a highly selective GC column for optimum analysis. There are a number of application notes available on this topic, highlighting the adherence to EPA methodologies, including EPA 8260, using a Zebron-624PLUS GC Column.
The analysis of semi-volatiles is a growing area of concern, particularly within environmental analysis, as semi-volatiles pose a threat to both environmental and animal well-being. In two SelectScience webinars, find out how volatiles can be analyzed in the soil using GC-MS, and also the best practices for the detection of both volatiles (VOCs) and semi-volatiles in water. In each case, we explore the best practices and benefits of using a single GC-MS platform for analysis.
Food quality
Chromatographic techniques are employed when testing whether food and beverages are safe for consumption and analyzing their components. This application note highlights how low-level odorants in wine can be identified using automated high-capacity sorptive extraction with GC-MS.
The Zebron™ ZB-5MSplus GC column by Phenomenex Inc., designed with a rigorous fused silica deactivation process for improved inertness, is recommended for polycyclic aromatic hydrocarbons (PAHs) testing in drinking water.
In a SelectScience video interview, Dr. Robert Trengove, of Murdoch University, Australia, highlights his team’s crucial work in screening for pesticide residues in human breast milk. By optimizing GC-MS/ MS analysis for human breast milk, the team has successfully increased the number of pesticides that can be simultaneously screened to 88. The presence of pesticide residues in breast milk is considered a matter of huge concern for the health of the child.
Pesticides
The prevalence of pesticides in the environment is of grave concern for consumers and environmentalists alike, as many tend to bioaccumulate and not only persist in locality but also move in bodies of water.
Whilst some of the most damaging pesticides have already been banned, it is vital that analysts have highly accurate methods for the detection of pesticides in soil, groundwater and fresh water – as well as food samples.
This application note shows how to improve results for GC-ECD analysis of chlorinated pesticides. This is of particular importance given that the fact that chlorinated pesticides are carcinogenic and have a high level of environmental persistence – and whilst many of them are now illegal to use, organochlorine compounds manufactured in the US are still a potential source of pesticide poisoning.
In this application note, JetClean Self-Cleaning Ion is successfully applied to the analysis of pesticide residue in order to increase productivity when analyzing by GC-MS and LC-MS. This makes a significant difference by reducing the need for manual source cleaning and increases the sample throughput of the GC/MS system.
Additionally, this guide shows methods for monitoring halogenated pollutants in a wide variety of matrices such as soil and animal tissue, using GC combined with electron capture detection. Restek’s Rtx®-CLPesticides columns are used, making analyses easier and enabling multiple environmental tests, such as PCB analysis, on the same set-up.
We are seeing industry-specific solutions that meet and exceed system suitability requirements. For example, the ZB-FAME GC column from Phenomenex provides complete separation of fatty acid methyl esters (FAMEs) in food in less than 11 minutes, while traditional columns take an hour long to separate these components in food.
Dr. Ramkumar Dhandapani Phenomenex
5. Current and future trends
Gas chromatography remains a mainstay technique for many labs.
Over recent years, we have seen many exciting column developments from the cage-like design of Agilent Intuvo columns – we also expect to see an increase in application specific columns, making it easier to choose the best column for your needs.
Gas chromatographic systems are becoming more automated, a trend expected to continue as labs move to being smarter, which will be aided by machine learning troubleshooting models. This can already be seen on the Agilent Intuvo and 8890 models which alert the analyst when preventative maintenance needs to be carried out, thus enabling simpler maintenance solutions and reduced downtime.
We expect to see a rise in greener chromatography, with many labs looking to reduce the carbon footprint of their activities.
The rise of interchangeable consumables will simplify choice, for example, Thermo Trace 1300/1310 and PE Clarus 590/690 GCs both use consumables that are interchangeable with most Agilent GCs.
Lower detection limits are anticipated across the board from columns, as are systems with a need for increased inertness and cleanliness.
With increasing regulation across the sector, the development of simple and automated gas chromatography will be seen over the years to come. As you look to purchase your next GC column or system, be sure to consider its environmental impacts - we expect to see a rise in greener chromatography, with many labs looking to reduce the carbon footprint of their activities.
6. Summary and acknowledgements
Many factors, ranging from dimensions to analyte properties, can influence your choice of a GC column.
By reviewing the library of published findings and applying a few simple considerations, it’s easier to select the correct column and get reproducible data.
With the growth in application areas for GC, there will be a rise in ready-to-use GC columns for your experiments. Visit the SelectScience product library to find out about the latest GC systems and columns from leading manufacturers. When deciding to purchase, be sure to explore our user reviews and request more information about a product of interest. Stay up to date with GC methods and free application notes by visiting the SelectScience application note library.
Acknowledgements
We would like to recognize the following contributors for their invaluable input:
- Dr. Mark Badger, GC Accessories PMM at Restek
- Dr. Ramkumar Dhandapani, Global Product Manager – Gas Chromatography at Phenomenex
This issue, last reviewed on July 16, 2020





