Sustainable antibodies: How can labs go animal-free?
A team of experts at Merck KGaA, Darmstadt, Germany, discuss their sustainable, animal-free, recombinant IgG antibodies, and reveal why these could soon become a laboratory must-have for most researchers
23 Aug 2022

In this exclusive interview, we speak with a panel of scientific experts, Terri Borree, product manager, Sinead McManus, R&D manager, and Dr. Michael J. Moehlenbrock, immunoassay sales specialist at Merck, to find out about their pioneering, animal-free, recombinant antibodies, and learn how they are becoming a laboratory must-have to replace conventional native mouse antibodies. Our team of experts also highlight the importance of sustainability, and explore why these antibodies are standing out from the crowd, and how they are contributing towards building sustainable labs to ensure scientists can conduct their research without the need for animals across most disciplines.
Making the transition to non-animal derived antibodies
A recombinant antibody is a fragment that has been produced via recombinant coding genes. These synthetic monoclonal antibodies developed by recombinant DNA technology are generated outside the immune system using synthetic genes, without the need for animal immunization. Due to their exceptionally high specificity and sensitivity, recombinant antibodies are now extensively used in many scientific disciples, and have several advantages compared to their monoclonal counterparts, such as large single batch culture capability, without the need to use large animal populations. As science continues to advance at an increasing rate, so does the technology behind the development of these specialized recombinant antibodies – rendering them likely to take over as a primary method to produce monoclonal antibodies, without the need for host animals.
Although many research laboratories do not partake in animal testing, several institutes continue to use animal-derived components. The specialists at Merck have created their own novel technology to develop recombinant mouse IgG antibodies, B1 and B2, designed to reduce the possibility of false positive and negative test results in clinical assays arising from interfering antibodies in human sera.
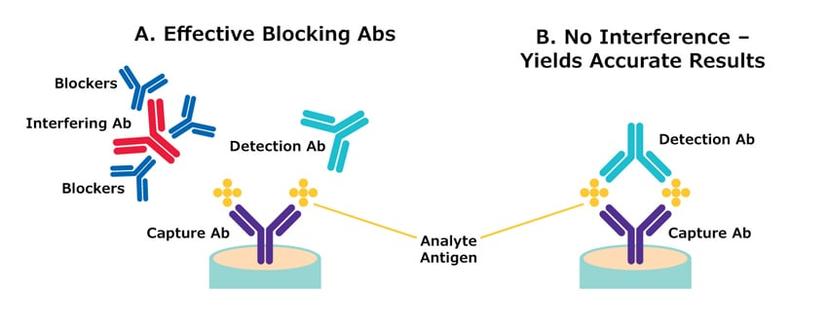
To find out more about the recombinant mouse IgG antibodies and supply chain difficulties, we spoke with Terri Borree, product manager at Merck, “I am responsible for antibody portfolio oversight in support of in vitro diagnostic manufacturing – which includes launching our recombinant mouse IgG antibodies designed to passively or actively target and remove interfering antibodies in clinical test samples. If an assay manufacturer develops a diagnostic test, they want to minimize false positive and false negative results. Therefore, we focused on helping manufacturers best remove those interfering substances present in a serum or plasma,” explains Borree. “Customers use blocking antibodies, like mouse IgG, to bind and remove excess nonspecific serum antibodies, leaving only analyte-specific antibodies behind, if present. This optimizes the accuracy of test results. Borree continues, “during the COVID-19 pandemic, Merck, like many reagent manufacturers, encountered supply chain difficulties, as conventional mouse IgG antibodies are animal-derived proteins, and reliant on the availability of tremendous amounts of mouse serum. Mice are very small and extracting enough serum to supply heavily used seasons, is problematic,” says Borree. During the pandemic, there was high demand to develop highly accurate diagnostic tests in significant quantities, and with that came the need for large single batches of blocking antibodies like mouse IgG leading to supply chain instability.
Lab sustainability is also becoming an increasing popular topic. Labs aim to conduct cutting-edge research, while implementing practices to minimize their impact on the environment by reducing energy consumption and conserving resources. “Merck stepped to the forefront of life science to make sustainability a corporate pillar of our company. It is our mission to find sustainable ways to deliver biological materials for IVD assay manufacturers that not only reduce our carbon footprint and the use of animals, but improve on them at the same time. If you consider mouse IgG, we require hundreds of thousands of mice to meet our production needs. We had the internal technology and scientific know-how to produce engineered antibodies, and we wanted to find a way to make this complex IgG molecule in a sustainable way. This was the foundational reason to create recombinant mouse IgG antibodies, not because the products on the market were inferior, it was because we wanted to do this without using animals,” explains Borree.

Dr. Michael Moehlenbrock, immunoassay sales specialist at Merck, also shares his thoughts, “my current role is serving as a manager of the business development team. We are focused on developing deep partnerships in technical developments with diagnostic manufacturers, working in critical workflow areas of immunoassay and genomics” says Moehlenbrock. “The current pandemic has put great strain on raw material supply chains for our customers, as it has for many industries.” Since the diagnostic market was heavily impacted by the COVID-19 pandemic, supply chain did experience many setbacks. These setbacks included the availability of raw materials for diagnostic assay manufacturing, such as mouse IgG, surfactants, supporting buffer salts, chelators, and critical enzymes. “Recombinant mouse IgG differs from native serum-derived mouse IgG in its ability to provide a sustainable, robust, and animal welfare conscience alternative to one of the most pressured and ubiquitously used blocking agents in diagnostics immunoassays. The antibodies at Merck are fully recombinant and culture-based, which protects the material from not only supply disruption, but the cost flux that can occur for serum-derived products based on the global market for mouse serum,” explains Moehlenbrock.
Sinead McManus, R&D manager at Merck, leads a team of experts who are developing, optimizing, and transferring new diagnostic antibodies for IVD manufacturing. McManus explains, “recombinant mouse IgG is a novel, sustainable replacement to serum-derived mouse IgG, providing manufacturers of immunoassays a more tightly controlled, reproducible product, while eliminating animal use. Additionally, we can ensure a more secure supply for our customers because we have better control of the raw materials and production specifications in-house,” shares McManus.
Next level in recombinant antibody technology
The team at Merck use their own innovative technology to manufacture the recombinant mouse IgG. “The recombinant mouse IgG B1 product is a blend of antibodies designed to mimic the IgG purified from native polyclonal mouse serum, a passive antibody blocker. Recombinant mouse IgG B2 is formulated like B1 but contains an active blocking component. These products are recombinantly manufactured and therefore completely animal free,” explains McManus.

Speaking directly about the technology, Borree shares, “we were able to study the mouse IgG molecule and select portions of it to engineer an optimized molecule. The two recombinant mouse IgG formulations are proprietary blends of a clone mix designed to mimic the blocking capabilities that mouse serum-derived IgG antibodies provide. They generally operate in the same manner as their native derived antibody counterparts but we created the B2 formulation with a twist. What we were able to do is not only create an antibody as a passive blocker to mimic serum-derived IgG in the B1 formulation, but also provide an enhanced version that actually targets unwanted IgG and extracts it with the B2 formulation. We produced two formulations to give diversity of selection for our manufacturing customers, whether they want to test B1 or B2, or run a comparison study on both the options are available to them”.
The global and competitive blocking antibody market size is vast and estimated to increase. Borree explains what sets their antibodies apart from the rest, “we are no longer reliant on pooled lots of serum from animals, and we can now use our clonal cell culture instead. This maintains product consistency, and a more secure supply to the customer. We’re removing that variability in the base of the product, which is the mouse serum. I would suggest this is one of the first animal-free mouse IgG blocking antibodies on the market. We have been able to create the optimal mouse IgG molecule by engineering it in cell lines, while providing two options, one that is designed to mimic, and the other one to enhance blocking abilities.” The recombinant antibodies at Merck are ethically sourced, and highly purified, “these are cell-cultured materials, so they maintain high consistency and a high level of purity, making them an ideal and sustainable material,” says Borree. McManus also shares her expertise, “by eliminating animal use and being a more tightly controlled product, the recombinant IgG antibodies provide manufacturers with an ethically sourced blocking solution to efficiently reduce nonspecific antibody interference in diagnostic assays. They can also improve lot reproducibility and test accuracy, while easing import restrictions.”
The recombinant IgG antibodies have already generated positive feedback from customers and could soon become a laboratory must-have. “We’ve received early feedback that states the product is a valuable replacement to animal-derived mouse IgG,” explains Borree. “The feedback thus far is that this recombinantly produced material performs as good, if not better, than native mouse serum derived IgG on a per mg basis. We would love to partner in a greater volume of manufacturing protocols with a more diverse set of diagnostic assays to demonstrate blocking performance, sustainability, robust supply, and cost control, which are impactful values for our customer base,” explains Moehlenbrock.
Impacting the future
Looking ahead, our experts reveal what impact these antibodies could have in the future, “sustainability is a key focus for us. Globally, many companies are looking for opportunities to reduce the use of animal-sourced materials. I believe this product opens the door to focus on those sustainable opportunities. We are looking to provide material that doesn’t change the cost of the assay, but one that can meet sustainability objectives,” explains Borree. Moehlenbrock also shares his outlook, “the impact is these antibodies provide an example of the evolution of manufacturing high-volume, animal-derived critical raw materials, as a more sustainable and animal welfare conscience alternative. This evolution of biologic manufacturing will not only have a tremendous impact on animal welfare but will also benefit the target market in providing a robust and scalable source of this and other raw materials used in high volumes within diagnostic manufacturing.”
The Life Science business of Merck KGaA, Darmstadt, Germany, operates as MilliporeSigma in the U.S. and Canada.