Addressing the top 3 challenges in cell line development: Clonality assurance, regulatory-compliant data, and high product quality
8 Aug 2022
Whether the cell line being developed is to produce a biotherapeutic (i.e., recombinant protein or monoclonal antibody) or the means to study and develop a biotherapeutic (i.e., used as a disease model or in drug screening), generating stable lines is an indispensable step.
As part of IND submissions and progress to the next phase of the pipeline, regulatory authorities require substantial documentation of the methods used during cell line development (CLD). Often, labs using manual record-keeping, such as those that utilize spreadsheets, find it challenging to demonstrate clonality with confidence. The need to provide all datasets including images accompanied by an audit trail required in GMP settings only adds another level of difficulty to an already tedious, labor- and time-consuming process.
Adopting the right technologies can accelerate and streamline the CLD process by eliminating the critical bottlenecks common in process development and biomanufacturing.
Today, we speak with a team of experts at Advanced Instruments to learn how labs can incorporate different instruments at specific points in the CLD workflow to significantly improve timelines and provide confidence.
Addressing challenge #1: Clonality assurance
“Beyond the standard operating procedures and hands-on activity required to move a product forward, the biggest challenge that labs face in cell line development is providing sufficient evidence of the clonal line generation to the regulators with very little guidance on what they specifically require,” notes Mark Rothenberg, Associate Director of Application Sciences at Advanced Instruments. “Can they measure and confirm the cell line clonality throughout the process, from start to finish, without any doubt and provide the appropriate evidence that the regulators expect?”
To minimize variation in the final product being generated, regulatory bodies such as the US Food and Drug Administration (FDA) request the demonstration of clonality. “All products from cell lines need to have evidence of clonality. Specifically, the FDA guidelines suggest either performing two rounds of limiting dilution to determine clonality or one round with image-based assurance,” says Emma Morris, Business Development Field Applications Scientist at Advanced Instruments, who interacts with customers regularly to deliver training and best practices.
Among the suite of technologies developed by Advanced Instruments, two systems in particular help achieve and easily document clonal assurance of a cell line – the Solentim VIPS™ and the Solentim Cell Metric® systems.
The Solentim VIPS™ offers a unique ‘double lock’ of clonality assurance, providing two pieces of evidence of clonality required for regulatory submission. The first assurance step provides image evidence of single cell seeding following dispensing. During the second assurance step, high-clarity, whole-well imaging is preformed one to four hours later to confirm the presence of a single cell. Following this, clonal outgrowth is monitored with daily whole-well imaging to create a visual timeline that can be traced back to the single cell, confirming monoclonality.
Morris continues, “This eliminates any human errors introduced by manual limiting dilution methods that require pipetting low cell/well concentrations. With image-based assurance, credible and objective evidence can be gathered at multiple time points.” Additionally, as the system is both a cell seeder and an imager, it can combine high efficiency single cell printing with high contrast whole well imaging in the same instrument.
CLD groups that have optimized the manual limiting dilution method for clonality can also benefit from incorporating an imager that can offer clonal assurance. “In this case, if the lab prefers to continue with the limiting dilution method, using the Solentim Cell Metric® imager can provide one piece of evidence of clonality, which means, there’s no need to perform the second round of manual limiting dilution,” says Morris. “This can save valuable time for the lab.”
Transfection Characterization of cell lines Process development
- Culture cells
- Transfection
- Select high-value clones
Single-cell isolation and cell viability Clonal assurance Clone productivity screening and titer
- Optimize
- Scale-up
- Quality control
SPECIFIC
INSTRUMENTS Solentim VIPS™
ICON™ with
STUDIUS™ SPECIFIC
ROLE Single-cell seeding with in-well verification Evidence of clonality via daily whole-well imaging Digital data management
Each system offers a unique benefit to accelerate cell line development. To expand on the key differences between the two systems, and how labs can choose which one is more appropriate for their workflows, we ask Camilla Domeneghetti, Biology Manager – Cell Line Development at Advanced Instruments. “The VIPS™ is a single-cell seeder. It allows scientists to dispense a single cell into a 96- or 384-well plate and completely removes the need for manual limiting dilution methods. The images provided by the VIPS™ can be directly used for regulatory submissions,” she says. “The Cell Metric®, on the other hand, is a whole well imager that can monitor the colony growth across a specific time point. And again, it removes the need for manual processes. It can speed up scientists' workflows and provide metrics that can then be submitted as part of the clonality report.” The Solentim systems therefore provide a solution to accelerate your workflow and deliver confidence in results, regardless of the characterization stage you are working on, or the process you are using.
Addressing challenge #2: Data management and record-keeping
Another common challenge observed in cell line development is managing and documenting the copious amounts of data generated. “We’ve heard scientists complain about the number of spreadsheets they need to manage and review,” says Rothenberg. “When data management is laborious and tedious, it can introduce biases into the decision-making process, potentially influencing whether or not a clone moves forward and gets approved.”
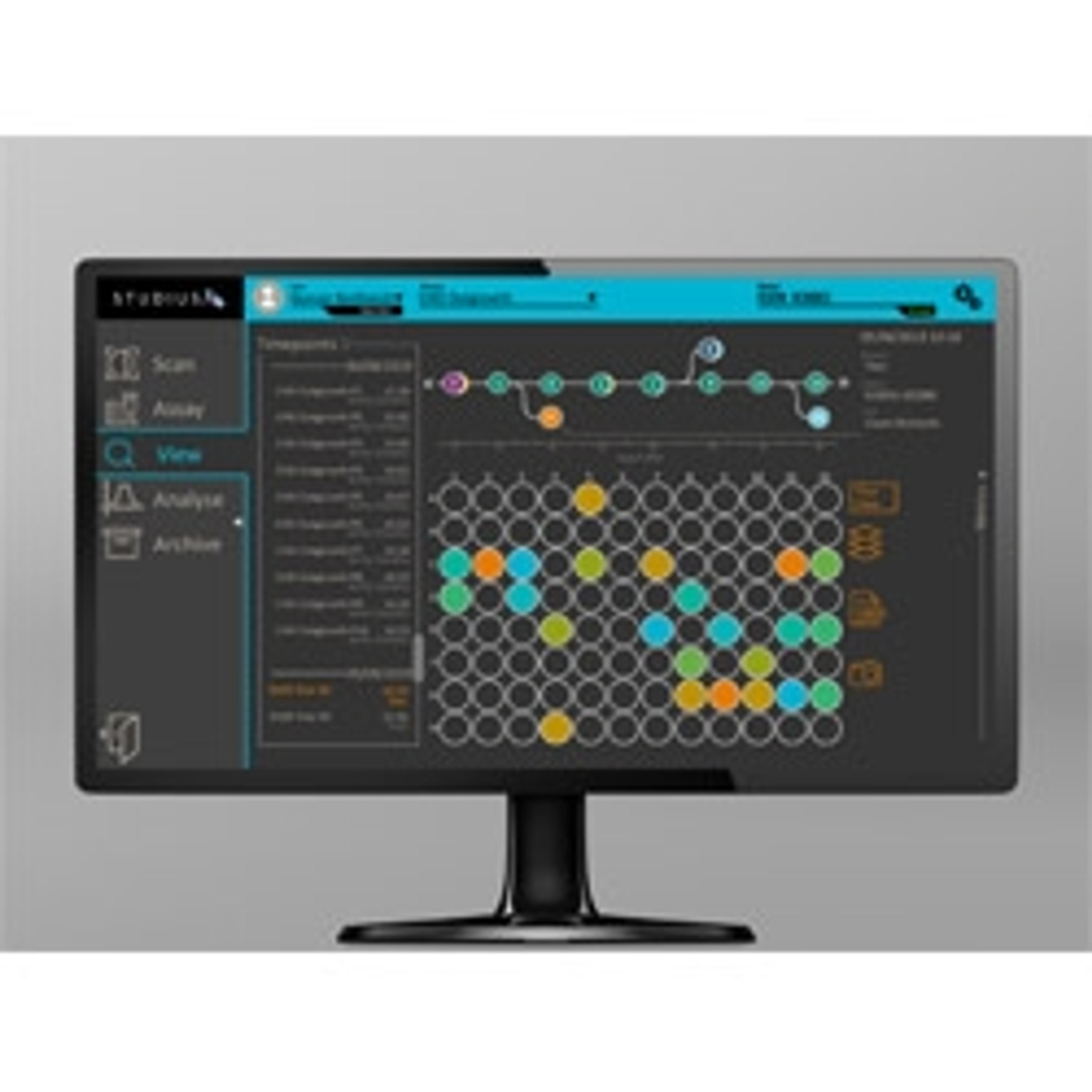
One way to alleviate data overwhelm is to adopt digital methods to capture, store, and manage datasets generated at different CLD stages. Not only is the data meticulously recorded for regulatory purposes, but it also enables trends and patterns to be uncovered, thereby enhancing the scientific decisions made by the lab.
Advanced Instruments' STUDIUS™ CLD data management platform incorporates data on clonality, confluence, titer, and cell viability together from VIPS seeding, Cell Metric clonal imaging and ICON’s titer and cell viability measurements . This allows analysis and decisions on best performing clones to be made instantaneously with minimal human input and without spreadsheets. The possibility for error is minimized and clones are tracked throughout the process from cell printing to final clone selection.
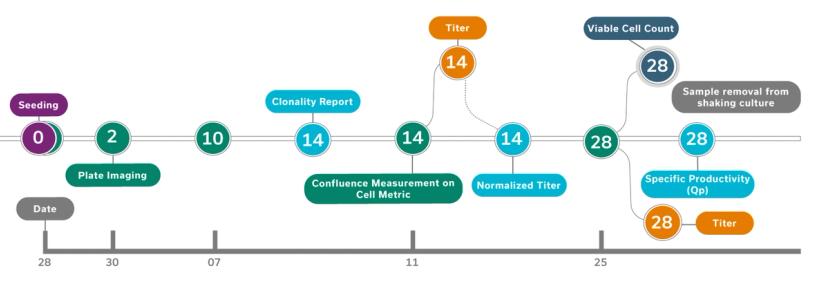
“The new ICON™ technology looks at critical cell quality attributes in samples, measuring for productivity or titer and viable cell density. It can be used in clonal expansion stages and also in fed-batch stages to streamline and rank top clones that will be eventually moved up to the bioreactance stages,” explains Domeneghetti. “The ICON is integrated with STUDIUS™ which documents the data, generates ranks according to user-defined parameters, and tracks the clones from seeding to selection.”
Addressing challenge #3: Upholding high product quality from start to finish
Finally, when the goal is to achieve a successful IND submission, upholding and monitoring best practices at every step is of utmost importance to ensure that the final product is of high quality. Whether it is monitoring the cell culture media to obtain optimum yields or monitoring the cells themselves to promote growth, these seemingly small variables in cell line development can have a cascading impact on the final product.

A metric often used to check cell culture media quality is its osmolality. “Osmometers have long been used in the clinical sector. It's now finding utility and importance in the biopharma market,” notes Rothenberg. “In a collaborative study with the UK Catapult Group, we looked at the impact of osmolality on AAV production. The results showed that increasing osmolality after transfection can increase the overall yield and quality of the AAV virus. There's also a growing volume of literature on this topic. Essentially, changing the osmolality affects the cellular health and production outcomes.”
Rothenberg continues: “To this end, using our Freezing Point Depression technology we’ve expanded our osmometer line to support the measurement of aqueous samples in the biopharmaceutical space. This unique line is designed for those in the GMP space and provides freedom to operate when osmolality is required. For instance, the OsmoTECH® XT measures the widest variety of sample types across multiple workflows, including cell and gene therapy. The multi-sampler, OsmoTECH® PRO, can measure up to 20 samples, to support increased sampling requirements. Finally, our brand new OsmoTECH® HT designed for high-throughput labs can measure osmolality in 96-well plates.”
Quality attributes related to cell growth and cell health also need to be maintained along the cell line development workflow, even after it advances to scale-up and manufacturing. “To help scientists develop high-value cell lines and optimize growth conditions, we have three different cell growth supplements for both CHO and HEK cell lines,” mentions Morris. “The InstiGRO™ is designed to improve single-cell growth, the InstiSHAKE™ supports your cells as they transition from static to shaking culture, and finally, the InstiTHAW™ offers support to cells throughout the cryopreservation process.”
To accelerate the overall cell line development workflow, and maintain data integrity and quality at the same time, labs will need to identify existing bottlenecks and source specific technologies, products, or alternative practices that minimize these time sinks. On the technology side, manufacturers, too, are focusing on advancing capabilities to speed up rate-limiting steps and offer more accuracy at every stage. As for what’s next for instruments used in cell line development, Rothenberg says, “We anticipate new technologies developed around improving functionality and real-time data tracking, as well as the ability to use AI and machine learning to help us make go/no-go decisions earlier in the process.”