Custom-Made Multiplex Assays Aid Unique Toxicity Tests in Agrochemicals
Multiplex biochemical assays, sometimes customized, help develop the toxicity profile and selection of agrochemicals before they enter the R&D process
7 Feb 2018

“In the toxicology center, we look at all the adverse effects of compounds that are currently in the R&D process,” says Blanck. “We sometimes also study compounds already in the market that need testing due to a regulatory change.”.
“We need to have a better idea of the different molecular initiative events behind the toxic response to endocrine disruptors,” says Blanck. Animal models are typically used to test agrochemicals, with any adverse effects documented. This approach, however, does not precisely reveal key molecular events between treatment and the toxic response. Blanck’s team hopes to obtain a clearer idea of the intervening developments that lead to this toxicity.
“For example, we use metabolomic approaches and look at differential expressions of some metabolites between control and treated groups,” explains Blanck. “We use human kidney cell lines that inherently express steroid hormones and look at fluctuations in hormone levels upon treatment with specific compounds that are being tested for steroid disruption. This gives us a toxicology signature of the compound.”
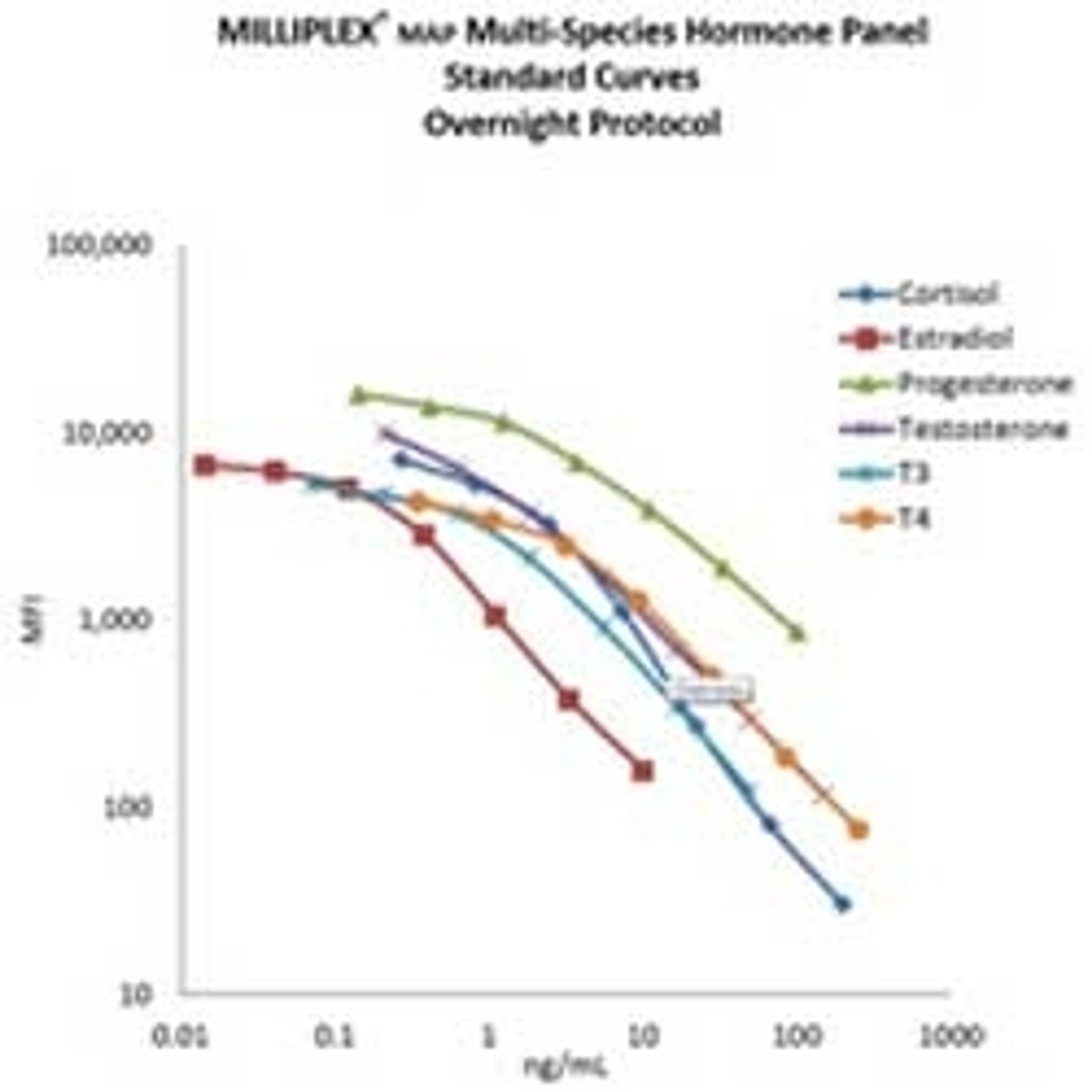
Blanck’s team has a portfolio of hormones that is measured onsite using multiplex assays. While ‘off the shelf’ assays can come in handy, Blanck often customizes these assays in collaboration with the Merck KGaA Custom Immunoassay team. “We wanted them to optimize the kits so they could better answer our questions,” says Blanck. “The standard curve for one of the hormones in an off-the-shelf kit was not optimal. It meant we had to dilute our samples, risking inaccurate quantification. The Custom Immunoassay team did a great job by optimizing the kit and working on the standard curve for this hormone. This enabled us to have a range of sensitivity without having to dilute the samples.”

Dr. Olivier Blanck and a colleague, Sandrine Pernot, perform the multiplex assay and examine data. Image courtesy of Dr. Blanck.
“We also had custom assays for four different hormones that weren’t available off-the-shelf,” Blanck continues. “This kit only had three analytes. When we asked if it was possible to have a 4-analyte kit, they did it.” One of the customized MILLIPLEX® MAP kits used by Blanck’s team measures thyroid hormones and thyroid stimulating hormone (TSH), while another measures steroid hormones such as estradiol, cortisol, progesterone, and testosterone.
“These two kits are very important for our endocrine disruption projects because we need accurate reporting of hormone levels. If you don’t have the kits that correspond specifically to your scientific needs, it can get very challenging,” Blanck admits. “Also, the MILLIPLEX® MAP kits go through extensive validation and have a strong batch-to-batch reproducibility.”
The MILLIPLEX® MAP multiplex assays, enable detection of multiple proteins in the same sample. “With multiplex assays, we can use a low volume of sample to measure multiple analytes. Multiplexing also saves time; if you have a lot of hormones to measure this would take too much time on a conventional biochemical assay,” says Blanck. “For us, something that increases throughput, reduces sample volumes, and decreases time is always interesting.”
With increased regulation of endocrine disruptors, it is possible that future studies will include detection of hormone levels in animal models in utero and in neonates, thereby making it very important to offer lower limits of detection. “I have started to have a discussion with Merck [KGaA, Darmstadt, Germany] in developing the kits that could do this,” says Blanck. “Also, we would like more assays developed so we can target a larger number of hormones and biomarkers of toxicity.”
The custom immunoassay team at Merck KGaA are experts at developing new assays alongside scientists such as Blanck. In the absence of a readily available assay, the team uses the customer’s samples to validate and develop multiplexing options for the analytes of interest. These assays are unique and tailored for each scientist. Soon after developing the customized endocrine disruptor assay with Blanck, the MILLIPLEX® MAP Multi-Species Hormone Panel became available for other scientists working on similar projects.
Learn more about Merck KGaA, Darmstadt, Germany’s Custom MILLIPLEX®MAP assay design here. Merck KGaA supports a variety of platforms including Luminex® instrumentation, and the new SMCxPRO™ High Sensitivity Immunoassay platform.