iPSCs Help Decipher the Debilitating Side-Effects of Chemotherapy
Dr. Eileen Dolan explains how she studies genetic variants that can predispose a patient to experience neuropathy after chemotherapy treatment
20 Aug 2017
The most common side-effect of cancer chemotherapy is peripheral neuropathy, a condition where nerves of the periphery, distant from the brain, are damaged. Such a neuropathy, causing numbness, hearing loss or a dramatic change in reflexes, can reduce the post-chemotherapy quality of life in cancer patients. In this interview, SelectScience® speaks with Dr. M. Eileen Dolan, Professor at the University of Chicago, who studies how genetics plays a role in predicting which patients are at greatest risk of chemotherapy-induced peripheral neuropathy.
“I’m most interested in individuals that are young adults or children, who receive chemotherapy, and have a life ahead of them. They deal with very serious side-effects of chemotherapy such as peripheral neuropathy, hearing loss or tinnitus,” says Dr. Dolan, who began her career by studying chemotherapy resistance before she began focusing on how genes can shape one’s response and toxicity to chemo medications. “Pharmacogenomics is a component of personalized medicine where the role of genetics is considered in identifying patients who are more likely to either respond positively to drugs or be at risk for a very serious toxicity.”
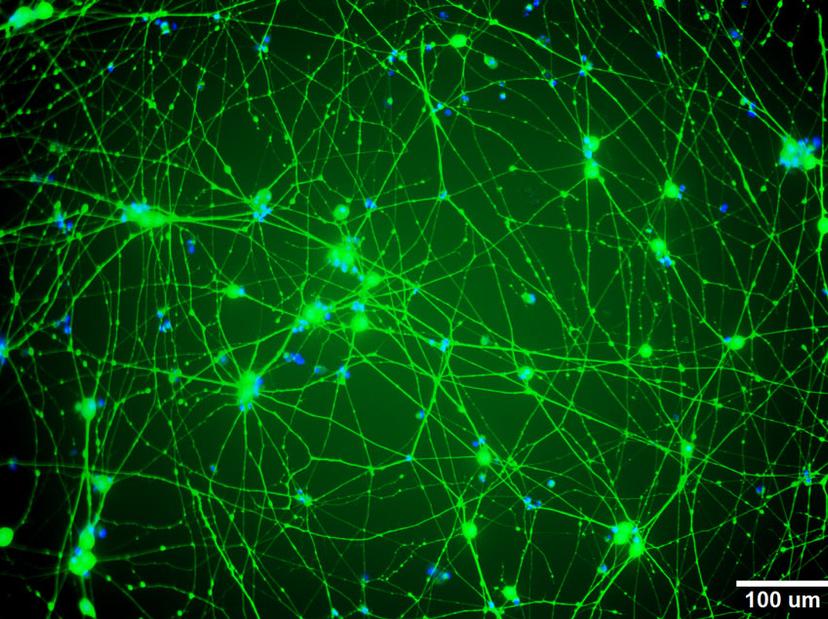
Chemotherapy-induced peripheral neuropathy occurs in about 30% of all cancer patients and, in many cases, is permanent. Young adults who have undergone cancer chemotherapy start experiencing symptoms of neuropathy within months of their treatment. Dr. Dolan’s lab studies the genetic variants that predispose patients to neuronal toxicity caused by chemotherapy medications such as cisplatin, vincristine and paclitaxel. “We functionally validate these genes and the genetic variants identified from clinical studies by using induced pluripotent stem cell (iPSC)-derived neurons,” explains Dr. Dolan. “We then use siRNA to knockdown genes in the iPSC-derived neurons to determine if genetic knockdown affects the neuron’s sensitivity to chemotherapy.”
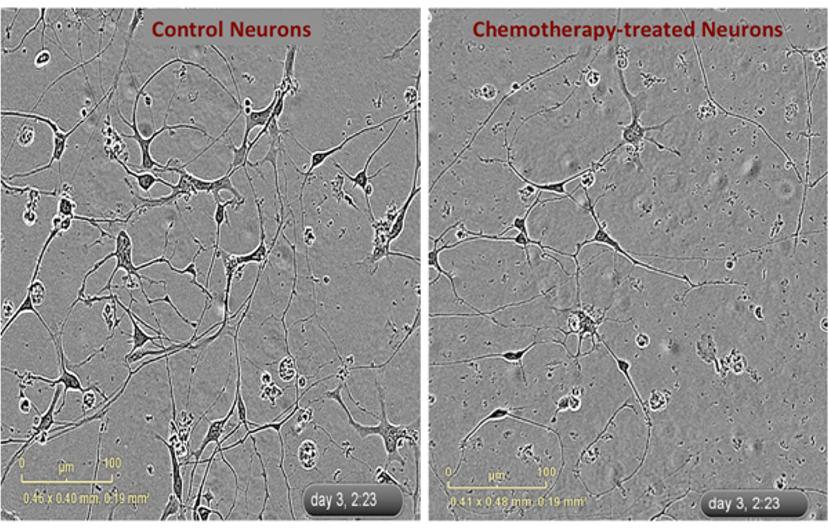
In this in vitro model of neuropathy in the Dolan lab, iPSC-derived neurons are plated and form neurites within a few hours. The team exposes these neurons, sometimes with genetic knockdowns, to mechanistically different types of chemotherapies and then evaluates the changes in neurite outgrowth. “The inhibition of neurite outgrowth is generally dose-dependent – the higher the dose of chemo, the more inhibition of neurite outgrowth you get. Also, there appear to be differences between mechanistically different chemotherapy agents. Some medications cause damage to the cells through apoptosis, while some others cause no damage to the health of the cell but exhibit a dramatic inhibition of neurite outgrowth,” says Dr. Dolan.

To understand neuropathy in iPSC-derived neurons, it is imperative to continually capture the neurons’ response to chemo treatments over time. Dr. Dolan’s team relies on live cell imaging for this information. “We set up the experiment in the IncuCyte and the images of neurons are taken. We not only get a dose response to chemotherapy but a time response as well,” notes Dr. Dolan. “This system also allows us to identify differences in the rate of neurite outgrowth upon chemotherapeutic treatment in the presence and absence of certain genes.”
“I love the amount of data that is generated over a few days,” says Dr. Dolan, commenting on her IncuCyte live cell imaging set-up. The mass of data obtained from this continuous live imaging then undergoes data analysis to look for signs of neuronal damage. “We can not only measure the length of the neurite, but can also measure the amount of branching and the thickness of the neurite. We look at a series of different morphological characteristics and try to identify what different changes are occurring as a result of exposure to different chemotherapeutic agents,” Dr. Dolan explains.
The significance of working with iPSCs is a strong potential for translational applications. “The plan in the future is to use patient-derived neurons and compare them with controls so we can unequivocally show that there are differences in neuronal morphologies between the two when you apply chemotherapy,” says Dr. Dolan. “Our hope is to use our data in a preclinical setting to screen new drugs and develop better treatments for cancer and prevent some of the debilitating side-effects of chemotherapy.”

