Osmolality testing for improved bioprocess development
Discover the benefits of harnessing the latest OsmoTECH osmometer technology for biologics manufacturing
15 May 2022

The manufacturing of biologics is a complex and costly process, requiring optimization and precise control of bioprocess parameters – all geared towards delivering the desired product quality and purity at the end of downstream processing. In this era of precision medicine, the industry is seeing a rise in the complexity and concentration of sample types, and the consequent need for robust analytical methods that provide more accurate and reliable measurements is greater than ever. Osmolality testing is a crucial parameter in bioprocessing which has a very broad range of applications, and its accurate measurement requires the best available technology.
In this article, we take a closer look at the role osmolality testing plays in biologics manufacturing and learn how the latest osmometers from Advanced Instruments are designed to overcome the challenges posed by innovative therapeutic formulations to help ensure their safety and efficacy for patients.
Process development and osmolality testing
Osmolality is one of the most important parameters for ensuring the quality of biopharmaceutical products. Its accurate determination using industry-standard freezing point depression technology has a very broad range of applications, from evaluating cell growth to ensuring active pharmaceutical ingredient purity and formulation components. It therefore has value right across the entire bioprocess workflow, from upstream to downstream and to the final formulation and filling stages.
One area where osmolality testing is particularly valuable is in process development. Here, it is used to optimize bioprocessing and analytical workflows in the development of a wide range of therapeutic modalities, from monoclonal and bispecific antibodies to synthetic medicines, and to ensure the compliance of these methods with the regulatory requirements of different jurisdictions.
Overcoming challenging sample types
The great thing about the XT model is that it can measure a broad osmolality range to cover all of our sample needs.
Will Heaton
DiscGenics
As the pharmaceutical industry moves towards more complex, higher concentration formulations, process development scientists are presented with samples that are more difficult to freeze and are incompatible with older technologies. These samples may include protein drug formulations, cryo-preservatives used in cell and gene therapy applications, or RNA therapeutics, and they can be highly concentrated and viscous. It is therefore critical to have an instrument that can measure such samples with optimal performance for reliable and accurate measurement, regulatory compliance, and ensures a final drug product is safe for administration.

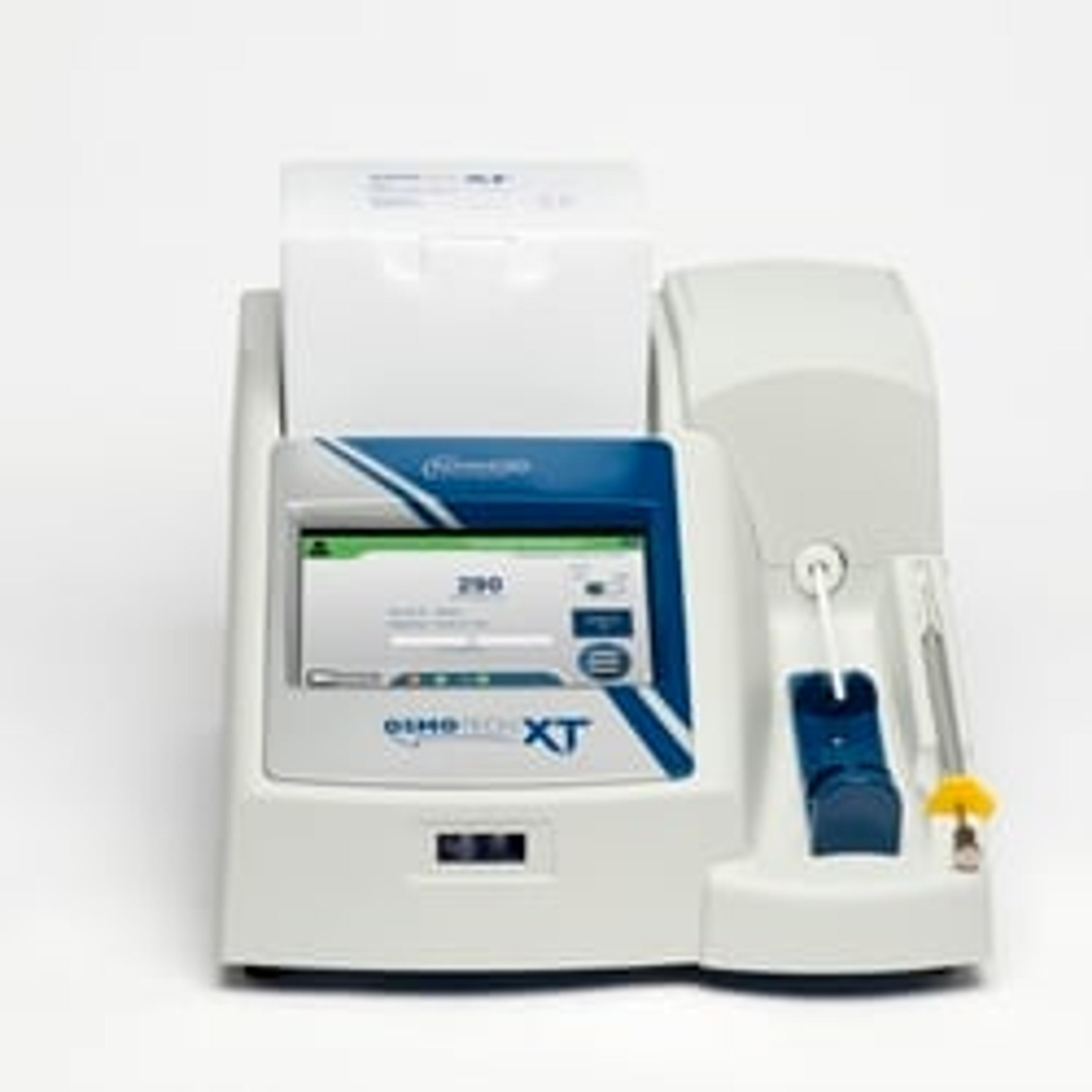
This is where the OsmoTECH® XT Single Sample Micro-Osmometer from Advanced Instruments, which uses new, intelligent freezing technology, can help researchers overcome testing these once tricky samples.
Bioprocessing method development for a variety of biologics calls for an osmometer with a wide range, and the OsmoTECH XT provides just that – up to an impressive 4000 mOsm/kg H₂O. In addition, one of its major benefits is the ability to handle the smaller sample volume of 20 μL, which provides a significant advantage from a sample management and control perspective, and can help with yield, especially in small batch production. Other osmometers have a sample volume of 100 μL, which may still sound small but often in fields such as cell and gene therapy, using the smallest volume possible becomes very important to achieve timely results, as well as to generate less waste.
Built for compliance
In today’s digital era, regulated environments such as biotech are increasingly required to manage and export data electronically in a computerized system. During process development, compliance to data integrity policies is therefore essential to ensure smooth technology transfer and scale-up under good manufacturing practice (GMP). The OsmoTECH XT represents an ideal replacement for older osmometer models from both a technology equivalency as well as a compliance perspective, whilst bringing an array of new features to comply with 21 CFR part 11, GMP and EU Annex 11 regulations and meet pharmacopeia osmolality testing guidelines. These features include different accounts with permissions set up separately to eliminate the potential of data manipulation, strengthened user security through remote locking, electronic signatures, and a comprehensive audit trail of all instrument events. All these features are specifically aligned with the recent focus on data integrity by the FDA and other regulatory bodies.
Technology adoption for osmolality testing
Osmolality determination is not a complicated test. However, it is a crucial control for drug product quality and a good instrument does make a significant difference. If you’re looking to upgrade your current osmometer, instrument selection should be based on the osmolality range across portfolio and modality, sample matrix, sample type – protein or non-protein – and the compliance requirements of the sites. Finally, good customer service is always important.
Take a look at what other researchers are saying about the latest equipment and technologies for osmolality testing. For example, Will Heaton, from DiscGenics, shared his opinion on the OsmoTECH® XT Single Sample Micro-Osmometer.

Learn more about how Advanced Instruments' OsmoTECH XT Single Sample Micro-Osmometer could help you.