End-to-end viral vector offerings propel AAV-based therapy forward
Viral vector experts share AAV-based gene therapy manufacturing challenges and solutions
20 Mar 2024
To meet increasing demands for robust viral vector solutions for AAV-based therapy manufacturing, Cytiva has recently acquired CEVEC Pharmaceuticals and integrated with the life sciences business of Pall Corporation.
Viral vectors are powerful tools that act as vehicles designed to deliver therapeutic genetic material directly into a cell. They have gained prominence in recent years, being utilized for gene therapies in rare diseases with high unmet needs and for administering COVID-19 vaccinations.
In this article, Dr. Markus Krieger, Vice President, Technology Development, and Clive Glover, Viral Vector Leader at Cytiva, discuss the challenges that the emerging field of adeno-associated virus (AAV)-based therapies is facing. They also share their thoughts on the methods used to manufacture AAVs and how co-optimizing components in the viral vector workflow may help solve a lot of the hurdles in the field today.
Overcome batch-to-batch variability in viral vector manufacturing
While viral vector platforms are increasingly being used to produce therapies across different disease areas, production of consistent and high-quality products using viral vectors remains far from perfect. A key challenge lies in the low productivity of the methods being used to manufacture them.
Transient transfection is a common method for producing AAV vectors, a leading viral vector platform used for gene therapy delivery today. This involves culturing cells to a final volume ready for transfection, which requires the addition of three types of plasmid DNA that need to combine inside the host cell for the host cell to be able to produce AAV. The step of transfection needs to be performed at every batch consuming large amounts of plasmid DNA. However, the process is fraught with cost-effectiveness and scalability challenges.
“There are existing supply chain hurdles in procuring GMP-grade plasmid DNA for industrial scale production, with wait times for delivery often extending beyond six months,” highlights Glover. “Moreover, the addition of the plasmid DNA within the bioreactor itself is not a process that lends itself to consistency, accuracy, or speed.” This results in immense batch-to-batch variability in terms of the end product. The inherent variability in the transient transfection process may be worsened further as transfection reagents tend to vary in quality, leading to small errors that compound over time.
These challenges are widely acknowledged in the industry today, to the point where the transfection process is not used for industrial scale production outside of AAV development. “Scientists have believed that making cells stably produce AAV – in a state referred to as a ‘producer cell line’ – would lead to cellular toxicity that would undermine AAV production at the scale relevant for clinical manufacturing,” shares Krieger. “The technical complexity of producing stable lines is the only reason transient transfection has been used for so long.”
Despite these compounding manufacturing challenges, the first generation of AAV-based gene therapies have opened up the option of life-changing therapies for patients with a high unmet medical need, and this approach remains a lucrative area of therapeutic research and development.
AAV-based gene therapies have opened up the option of life-changing therapies for patients with a high unmet medical need.
Collaboration driven by innovation
Positioned at the forefront of innovation in the viral vector space, the experienced team at Cytiva began looking for answers to address these seemingly disparate challenges across the AAV manufacturing workflow. They soon realized that the key was to co-optimize different problems by bringing advanced solutions together.
Cytiva’s first strategic step was acquiring the life sciences business of Pall Corporation. The formerly separated portfolios held disjointed offerings, with Pall offering bioreactors and filtration products and Cytiva offering chromatography solutions and cell culture media optimization. “With the merger, the Pall and Cytiva offerings quickly came together as full, end-to-end capabilities,” shares Glover.
The latest acquisition of CEVEC Pharmaceuticals in October 2022 then helped enrich Cytiva’s producer cell line development service. “We hope this will become the gold standard in AAV, as CHO cells have been with monoclonal antibodies,” explains Glover. “The combined portfolio provides the unique opportunity to co-optimize across the entire vector manufacturing workflow.”
The combined portfolio provides the unique opportunity to co-optimize across the entire vector manufacturing workflow
A robust and reliable platform to propel the field forward
For the expanding Cytiva viral vector team, boosting the productivity of viral vector manufacturing remains the big picture focus. “The experienced and innovative team we have inherited from CEVEC has been working away at this problem for years,” shares Glover. “We believe that the remarkable technology behind the ELEVECTA™ producer cell line that the team has developed will be key in boosting productivity by ten- or even hundred-fold.”
“In generating ELEVECTA™ stable producer cell lines for AAV production – our Cell Line and Process Development team can help manufacturers develop stable cell lines based on the requested serotype and gene of interest, with consistent batch-to-batch quality," explains Krieger.
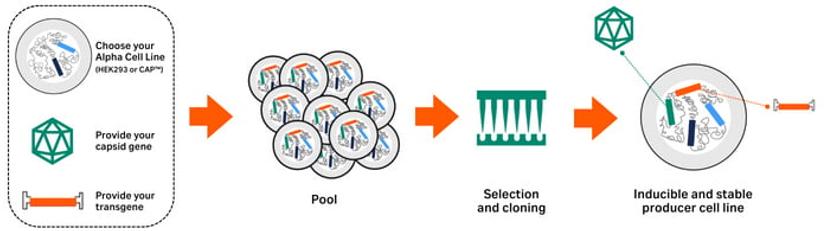
With a stable producer cell line, Krieger likens the yield and volume capabilities in AAV manufacturing to that with other recombinant protein production: allowing scalability up to the thousands of liters. This is a feat that is almost impossible with the commonly used transient transfection process. Furthermore, using stable producer cell lines eliminates a critical and challenging piece of raw material – the plasmid DNA – from the manufacturing process. This results in a more robust and consistent vector production process.
With production at a larger scale, the manufacturer has a better visibility on planning. Not having to depend on GMP-grade plasmid DNA, with its extended waiting times, batch planning becomes a lot and simpler and more reliable.
“My team works alongside the cell line development workstream, focusing on innovations that can help optimize the platform and improve productivity further,” highlights Krieger. “We are exploring both raw material optimizations, such as optimizing cell culture media for productivity gains, as well as process-related improvements to help shorten production turnarounds, enhance the cell line screening process, and more. We have even tested the ELEVECTA™ producer cell line in intensified perfusion mode, which has shown promise in increasing not just the scale but also the quality of the product.”
With access to the expanded Cytiva portfolio and ecosystem, Krieger is confident that his team is now better equipped to make quicker and more informed optimization decisions for their clients. “For example, we can enhance our optimized cell lines by consulting with the HyClone™ cell media development and manufacturing team at Cytiva – to develop an optimal medium,” he continues. “Having access to a broader team with wider expertise across the viral vector workflow will help drive innovation faster.”
With so much that yet remains to be discovered and understood about AAV biology, scientists are only just beginning to scratch the surface of what can be achieved. “Considering that productivity of the monoclonal field today has multiplied over an astounding hundred-fold since it first launched, I am certain a lot lies ahead for AAV productivity,” Krieger concludes.
Having access to broader team with wider expertise across the viral vector workflow will help drive innovation faster.

