The use of Analytical Quality by Design workflows in the pharmaceutical industry: All your questions answered
Catch up on this expert on-demand webinar to discover a novel approach for in silico method development and robustness assessment
6 Aug 2020

Are you curious about the potential of using an automated AQbD workflow across your analytical developments, looking to increase the efficiency of your method development work, or want to improve your regulatory communication for greater post-approval flexibility?
Good news – you’re in the right place!
In this popular webinar, now available on demand, Dr. Pankaj Aggarwal, principal scientist at Pfizer, is joined by Dr. Alexander H. Schmidt and Mijo Stanic, co-founders of Chromicent GmbH, as they provide a comprehensive account of how automated AQbD modeling can be used as a strategic approach across analytical operations of a large pharmaceutical company.
Read on for highlights from the live Q&A session or register to watch the webinar at any time that suits you.
Watch on demandQ: Is Dry Lab only compatible with Empower? Can it be used with Chromeleon or ChemStation?
DryLab's Empower Automation Module is only available for Empower 3, FR 2 or higher, and in combination with Waters instrumentation. There are currently no other chromatography data systems (CDS) that can seamlessly connect to DryLab. However, DryLab has been a standalone software since 1986 with no direct connection to a CDS, its modeling is based on imported chromatographic data of the .AIA/.CDF file-format which is available in most chromatographic data system such as OpenLab, Chromeleon, LabSolutions, ChemStation, etc.
Q: AQbD is a regulatory requirement – which regulators are interested in this design?
Regulatory environments such as the American, European and Japanese (and others) strive with mutual efforts in their ICH Initiative to move the industry towards an Analytical Quality by Design Approach. Guidelines and concept papers on topics such as Analytical Method Lifecycle Management (Q12) and Analytical Quality by Design (Q14) are available online on websites such as FDA.org and ICH.org.
Q: By running the experimental analysis of some working points using HPLC conditions (note: MODR was generated using UPLC data) do you think the MODR is still valid?
The MODR modeled in DryLab is valid for the phase system and the instrumentation from which the input data was derived. DryLab visualizes the design space and the MODR (the latter being the area in the design space that fulfills the method target profile, MTP). DryLab assesses the robustness of a method by investigating the model in regard to possible variations in the method conditions that can be expected in routine use, i.e., gradient sensitivity, temperature, pH and flow rate accuracy. The chromatographic interactions underlying the model can be expected to be the same for HPLC and UPLC as long as the phase system remains the same. System volume differences (such as dwell and extra column volume ) will be accurately modeled by DryLab, with the variations deriving from the differing instrument system (i.e., above mentioned differences in column heating accuracy, gradient sensitivity, solvent delivery accuracy, etc.) taken into account by altering the expected in-routine tolerances in the robustness module. In any case, every DryLab model is validated to substantiate the accuracy of its prediction.
Q: How is Drylab software better than Fusion in terms of DOE and design?
As DryLab is based on the absolute laws that describe retention mechanisms and chromatographic interactions, it can accurately model the whole of the design space—not just approximate the quality of a separation around a limited work point, as a statistical tool would do. This allows for a profound understanding of which parameters matter for the quality of the separation, enabling those which don’t to be downgraded in the reporting category. The DryLab model, once derived from the predetermined set of input runs, is accurately adjusted with only one click to change to the method’s parameters, for instance to the gradient (i.e. adding multiple steps) and column dimensions, without having to perform any additional runs. Being a scientific method development package, DryLab's DoE is at the center of its powerful modeling engine.
Q: For those who are new to AQbD, what is the best way to start AQbD for method development?
The first things to acknowledge are – what is the target and what is the goal you’re looking to achieve? Firstly, the analyst will need to gather as much knowledge as possible around the analyte. Then, you need to devise a systematic approach to answer the beginning questions. Documenting is important in this entire process.
Q: What are different types of data modeling that can be used for LC method development?
One approach is empirical modeling, involving collecting the data, modeling the design space, and devising an optimum condition. This can be combined with another approach called mechanistic modeling, which is DryLab's mechanism. The third approach is structure-based prediction (predictive modeling), using existing method information and compound information to make predictions based on physiochemical properties.
Q: How does using AQbD approaches for method development and ICH Q12 provide post-approval flexibility?
The term AQbD implies method development that is based on risk-based scientific understanding. By following an AQbD workflow you improve the communication between industry and regulatory agencies. For example, the knowledge management document generated by DryLab tells regulators how you developed your method and how robust the method is. This allows a better understanding of your method's performance region and therefore makes it easier to support post-approval changes without the need to revalidate. This document supports the requirements need to perform post-approval changes per ICH Q12.
Q: Where can I go for additional resources and questions regarding ICH Q12?
ICH guideline website and peer-review publications on AQbD, ICH Q12 and Method Lifecycle Management by Chromicent and Molnár-Institute.
Q: I believe that it would be more interesting to transfer the UHPLC method first to HPLC and then run the optimization tests using HPLC to generate the MODR. What is your opinion?
Regarding possible concerns about the success of a method transfer UPLC-HPLC or vice versa, the suggested approach would be to create a tGT/pH and a tGT/tC model for both instruments as an in silico comparability test, and then verify this as per a set of experimental runs. It is important to measure the dwell volume in both instruments as it has a strong impact on the retention properties of the separation. This approach will facilitate the method transfer.
Q: Is the database for method scouting public, or does it come with the software?
The database that quantitatively compares columns regarding their equivalency or difference and can be used for scouting comes with the software.
Q: How is solubility predicted? What parameters are used for prediction?
DryLab modeling predicts changes in selectivity based on the retention mechanism of HPLC and the chromatographic interactions observed in the input runs. DryLab does not predict solubility. However, there is a relationship between solubility and the accessible molecular hydrophobic surface area, which can be derived from RP-retention values.
Q: Is there any data or an in silico work model for upstream processes (cell culture processes with bioreactors) or cell-based assays?
Yes, please refer to Kochling et al. (2016) "A platform analytical quality by design (AQbD) approach for multiple UHPLC-UV and UHPLC–MS methods development for protein analysis" or reach out to info@molnar-institute.com for the publication.
Q: If we have DryLab software do we need to have fusion software to meet AQbD method development according to the ICH 8-Q11 guideline?
DryLab software has always been in full accordance with the mentioned guidelines, as its underlying modeling workflow follows what would later become mutual principles since DryLab's beginning (1986).
Q: When we apply the AQbD, do we still need to validate the method with all the parameters?
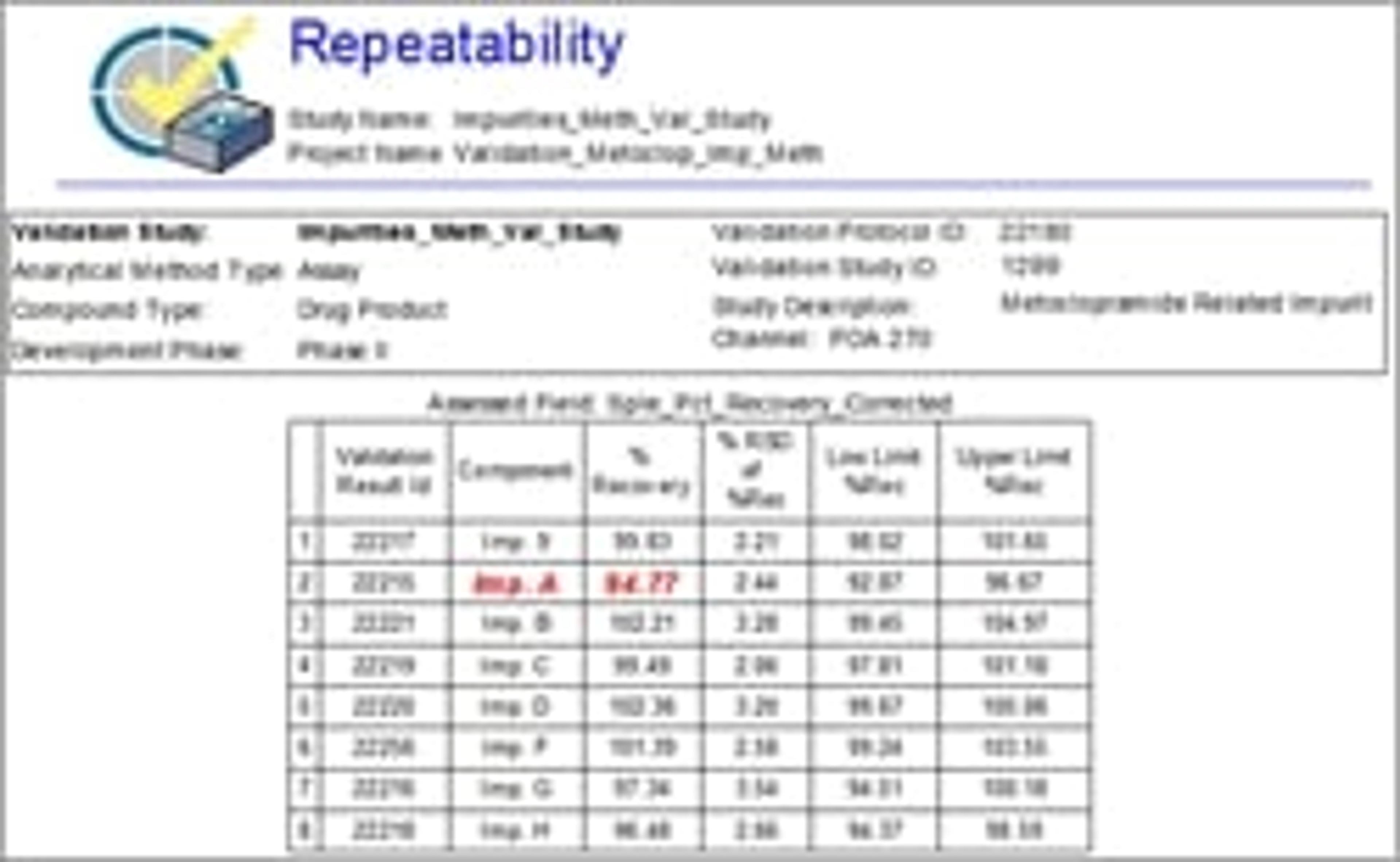
Every DryLab model is validated, however, due to its scientific and risk-based approach, only with a limited set of experimental runs to substantiate the accuracy of the model.
Q: Is DryLab limited to RPLC or are other chromatographic modes supported, too?
DryLab is an experiment-based universal tool, using the first-principle approach (linear solvent strength and solvophobic theory) to optimize peak spacing (selectivities) and critical resolution. Hence, it can be used in each LC-technique (RP, NP, HIC, IEX [salt-, or pH], HILIC) that applies non-covalent interactions between the phases (stationary and mobile), in order to separate the analytes (small or large molecules).
Q: Are there any application notes related to AQbD in pharma available?
Please refer to the more than 230 peer-reviewed publications, many of them featuring industry applications, as listed on the literature page of Molnár-Institute's website.
Q: Does the change of the method parameter require new validation?
Not if system suitability is met.
Q: Is there a dedicated tool in DryLab for column comparison?
Yes.
Q: Is a mass detector for peak tracking needed or is it possible to do this using only UV or fluorescence detector?
UV/FL detector is enough, but MS helps.
Q: How do you build the impact of different pH on different pKa of impurities into the in silico model?
By measuring the retention behavior.
Q: Can Empower and DryLab be used with just direct connection or does it work with terminal server connection too?
DryLab’s automation feature utilizes the Empower Toolkit and, as a requirement, needs Empower 3 FR2 or later for the connection to work. Depending on the type of environment in which DryLab will be used with the Empower CDS, we offer two types of connections:
- Direct connection to Empower – if DryLab and Empower are both available on the same workstation
- Automation service – if you would like to have more instances of DryLab to connect to the same Empower automation service, or if you want DryLab to connect to multiple instances of Empower within your network (Automation service requires administrator access and port sharing to work properly).
Citrix and other virtualization solutions should work properly, provided that Empower is available.
Q: Can the AQbD be applied to evaluate the GC analysis?
Yes.
Q: Is there a web page to find extra information about AQbD?
Molnar-Institute.com/DryLab and Molnar-Institute.com/Literature
SelectScience runs 3-4 webinars a month across various scientific topics, discover more of our upcoming webinars>>