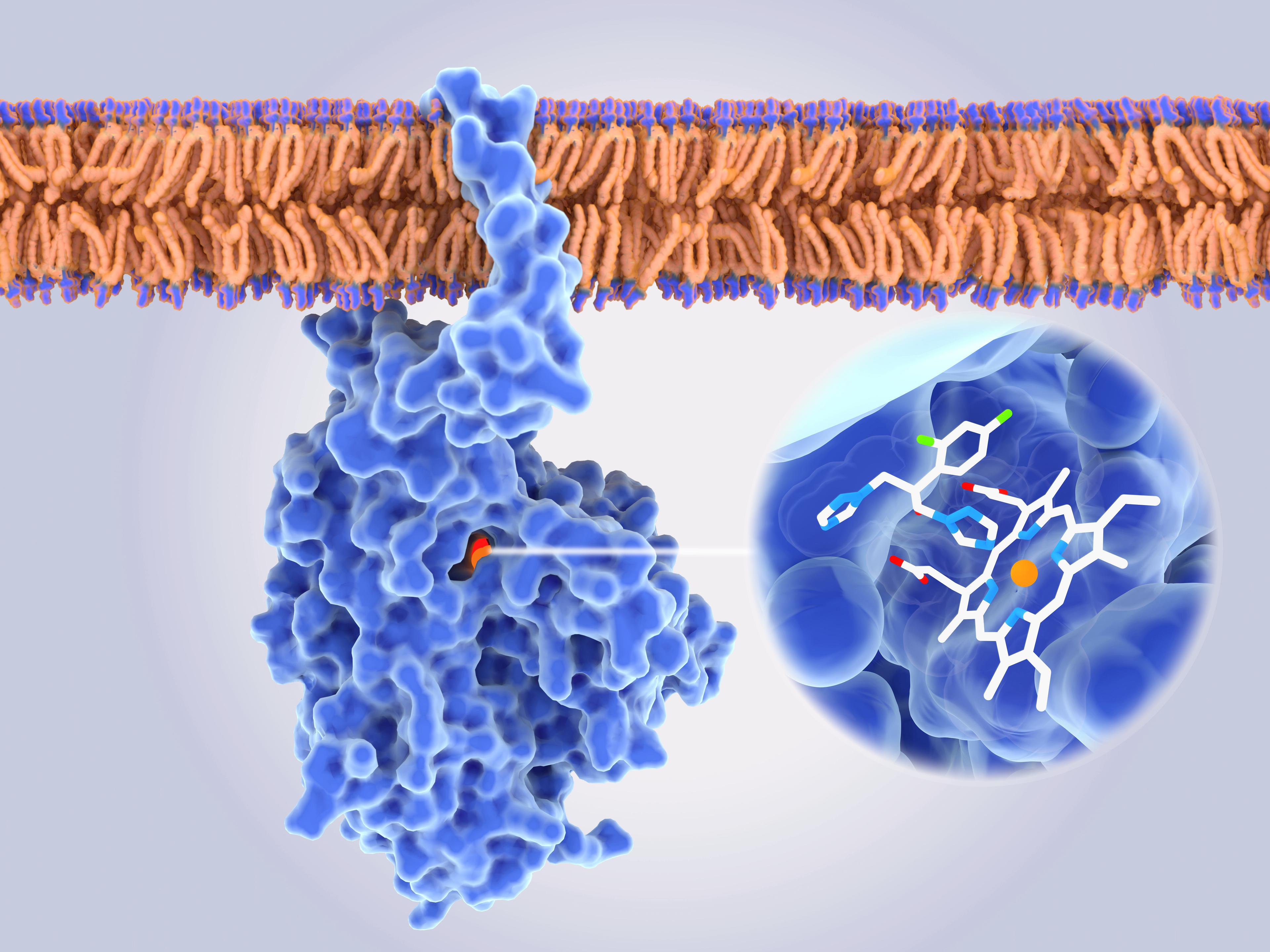
Pharmaceutical developments
Pharmaceutical development is entering a new phase, shaped by the growing need for more precise, efficient, and patient-centric approaches. As the industry responds to increasing complexity, from early-stage research to regulatory approval, there’s a clear shift toward more integrated, data-informed strategies.
Emerging technologies in formulation, digital health, and AI-driven analytics are helping to streamline development, improve decision-making, and support better therapeutic outcomes. From adaptive trial design to advanced delivery platforms, innovation is steadily influencing how medicines are brought to market.
This feature explores the evolving landscape of pharmaceutical development, highlighting expert insights, practical tools, and the trends guiding progress across the sector.

This guide delves into the essential elements of modern pharmaceutical practices, focusing on critical aspects such as data integrity, automation, analytical service and quality control, and the importance of service and training.
Download eBook
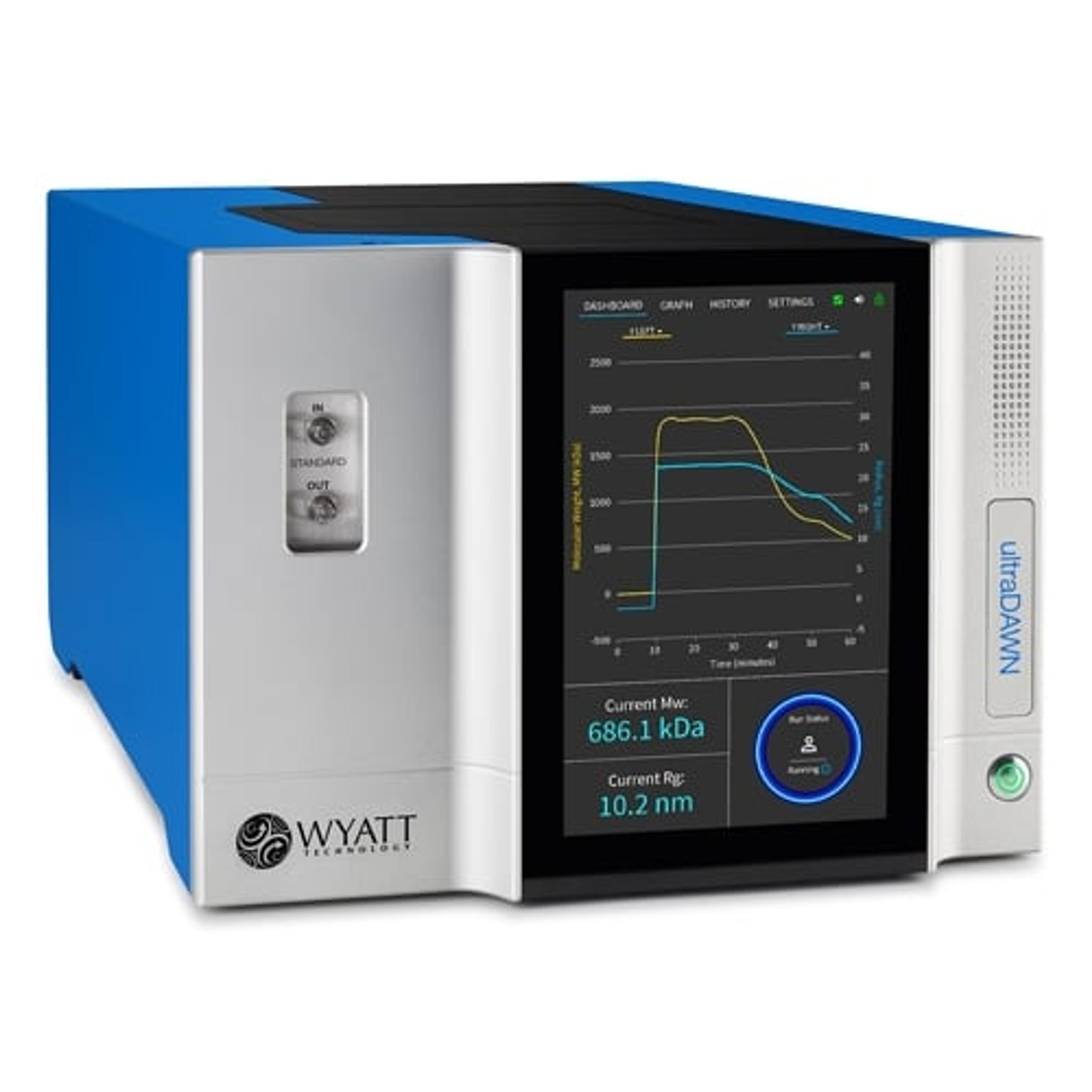
Explore how advanced light scattering enhances protein characterization in biologics, from candidate selection to formulation, process development, and QC, ensuring precision and performance.
Visit hub
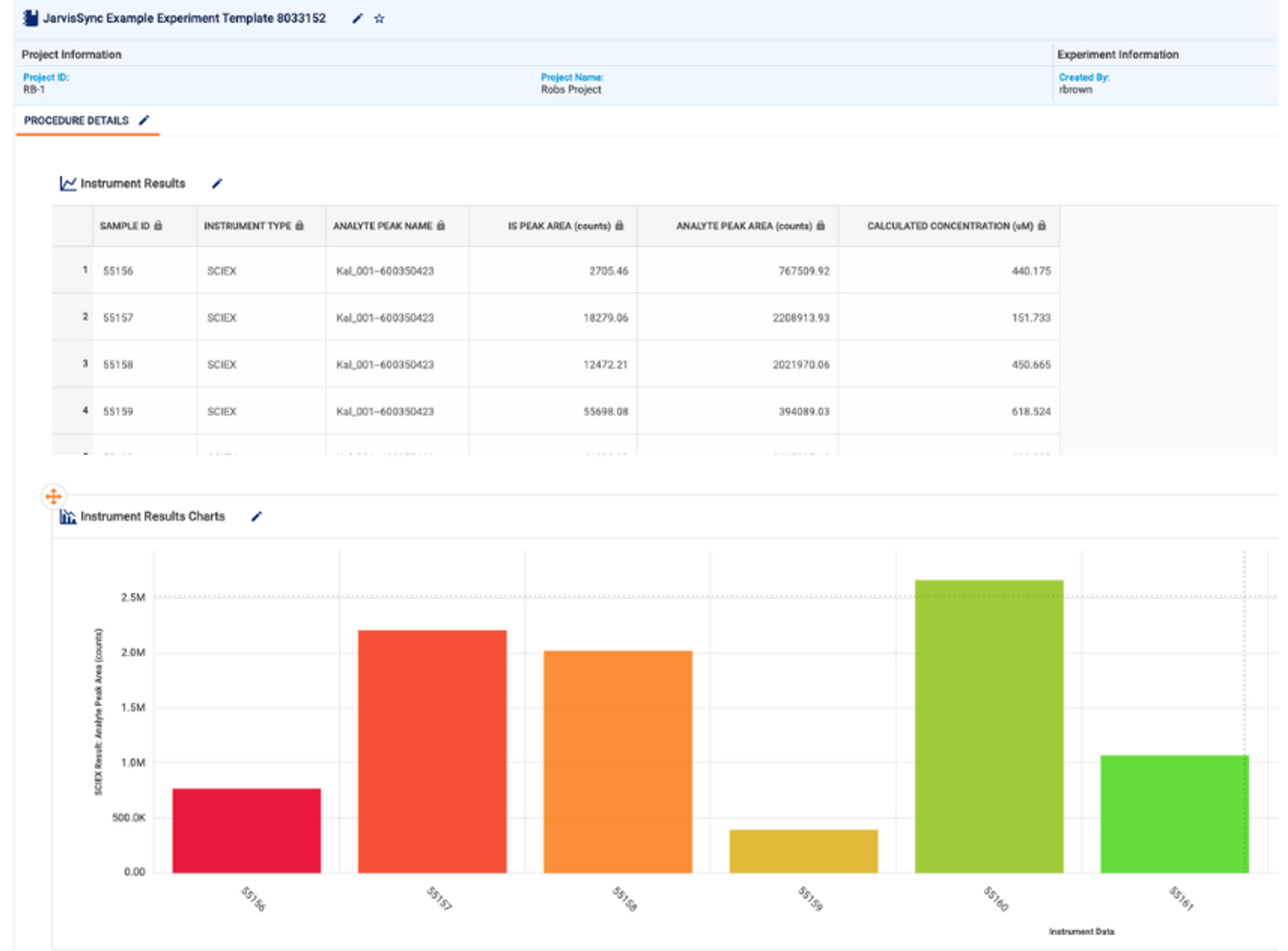
AI is helping biopharma companies speed up drug discovery and clinical trials, but fragmented data can slow progress. With real-world examples, see how digital transformation and AI automation are improving lab efficiency and scientific insight.
Download resource
Roche Diagnostics data demonstrates how the 2.1 mm YMC Accura BioPro IEX SF gives superior MS-coupled resolution on 4.6 mm PEEK in IEX workflows.
Download resource
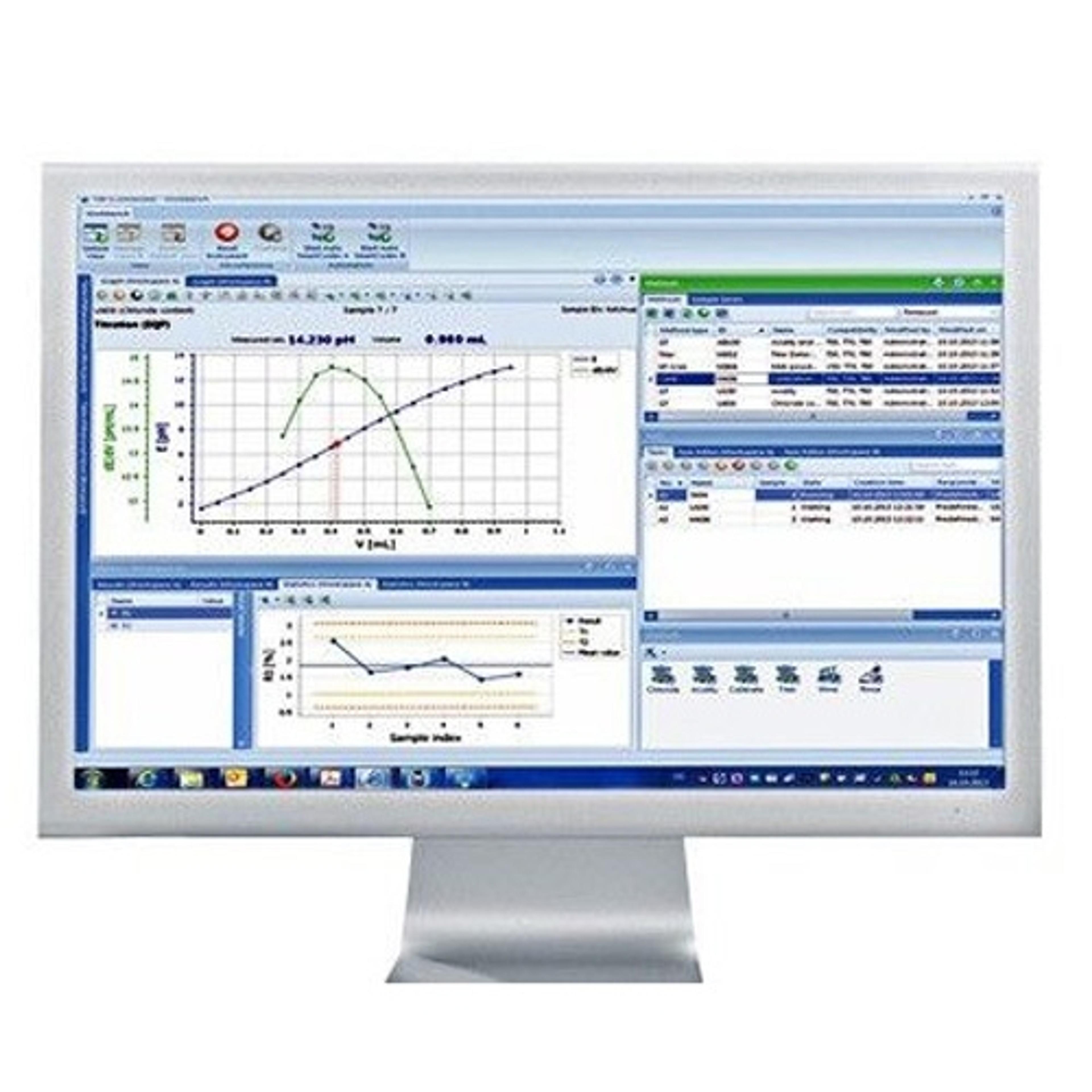
Learn how Good Measuring Practices support GMP, 21 CFR Part 11, and lab qualification across the full lifecycle of analytical instruments.
Download eBook
Explore how automation supports regulatory compliance in pharmaceutical labs, reducing manual errors, improving audit trails, and streamlining documentation for FDA and GMP readiness.
Download resource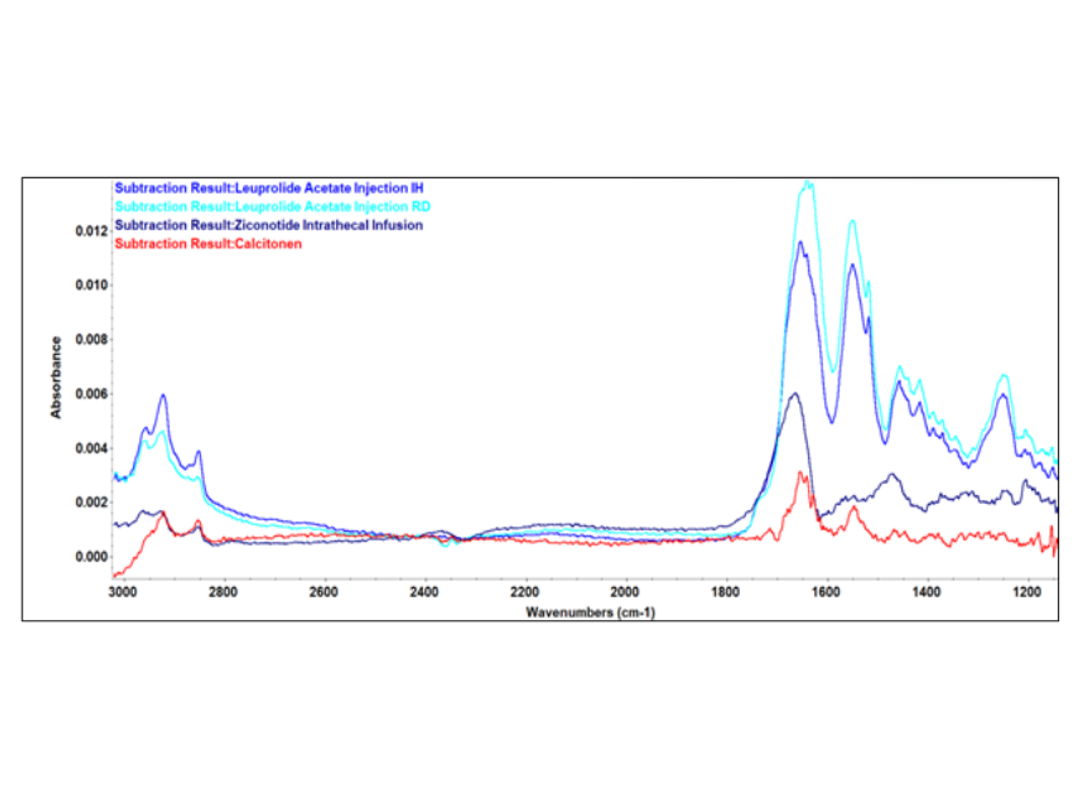
Learn how the Harrick ConcentratIR2™ enables clear resolution of amide bands in model pharmaceutical peptides ziconotide, calcitonin, and leuprolide acetate at concentrations relevant to early-stage formulation and quality control.
Download resource