2'F RNA CPG
CPG for the incorporation of a 2'-fluoro modified nucleobase at the 3' end of an oligonucleotide
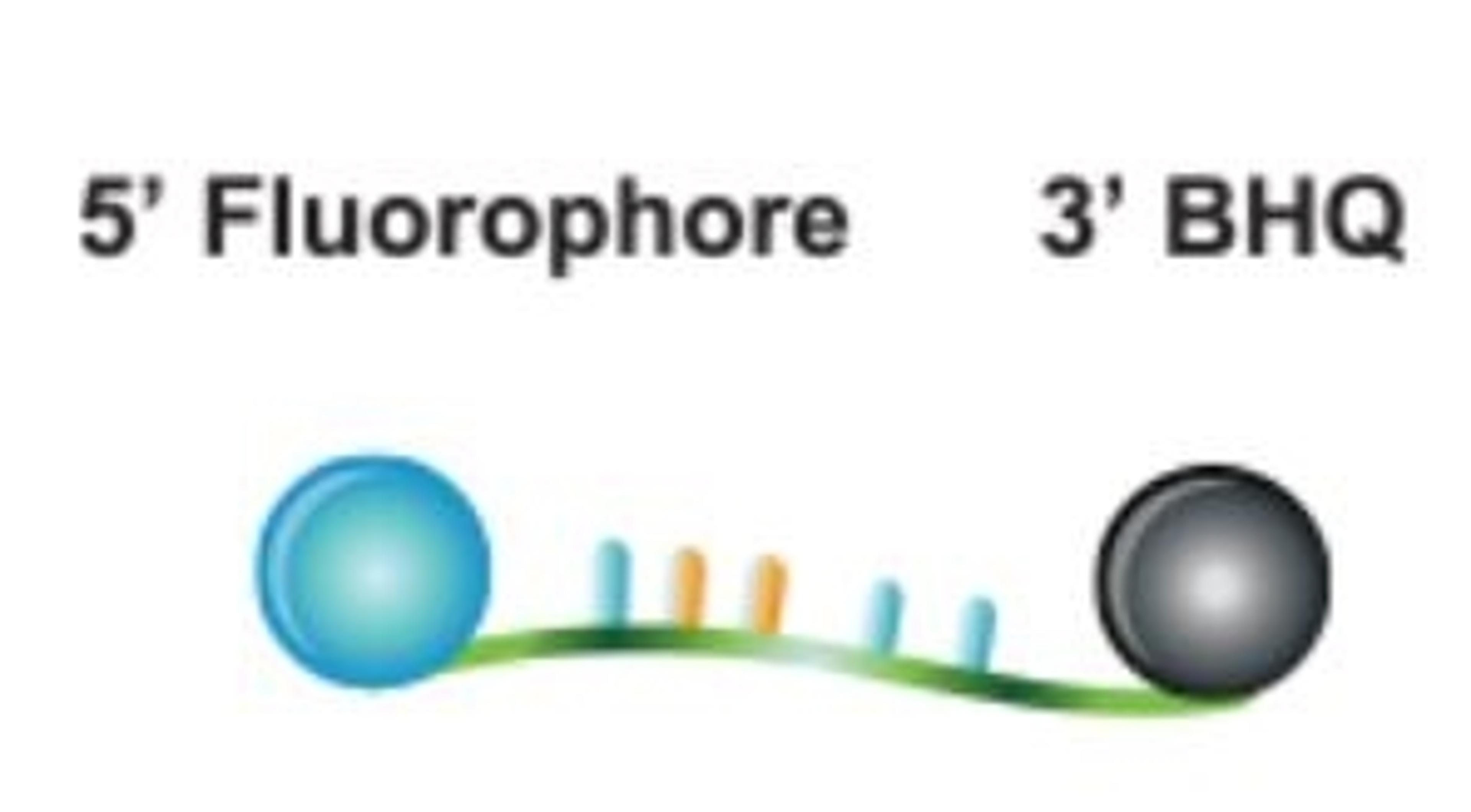
2'-F-RNA oligonucleotides adopt an A-form helix on hybridisation to a target. Whereas a hydroxyl group of RNA is a hydrogen bond donor, fluorine appears to be a weak acceptor. These features of 2'-F-RNA oligonucleotides lead to certain interesting properties. For example, it was demonstrated that oligonucleotides hybridise to a RNA oligonucleotide in the following order of increasing stability: DNA < RNA < 2'-OMe-RNA < 2'-F-RNA. (1) Aptamers composed of 2'-F–RNA bind targets with higher affinities and are more resistant to nucleases, compared to RNA aptamers. (2) In addition, 2'-F-RNA can be effectively used in siRNA applications. It has been shown that siRNA synthesised with 2'-F pyrimidine nucleosides are more inhibitory, and show considerably increased stability in human plasma, compared to siRNA. (3) 2'-F-RNA is now finding a number of applications, especially in RNA interference for the specific silencing of genes in cells and in vivo. (4) We provide a range of 2'-F phosphoramidites and CPGs, with a variety of pore sizes and linkers consistent with our unmodified DNA and RNA CPG products. The protecting group strategies are compatible with the usual DNA and RNA chemistries.