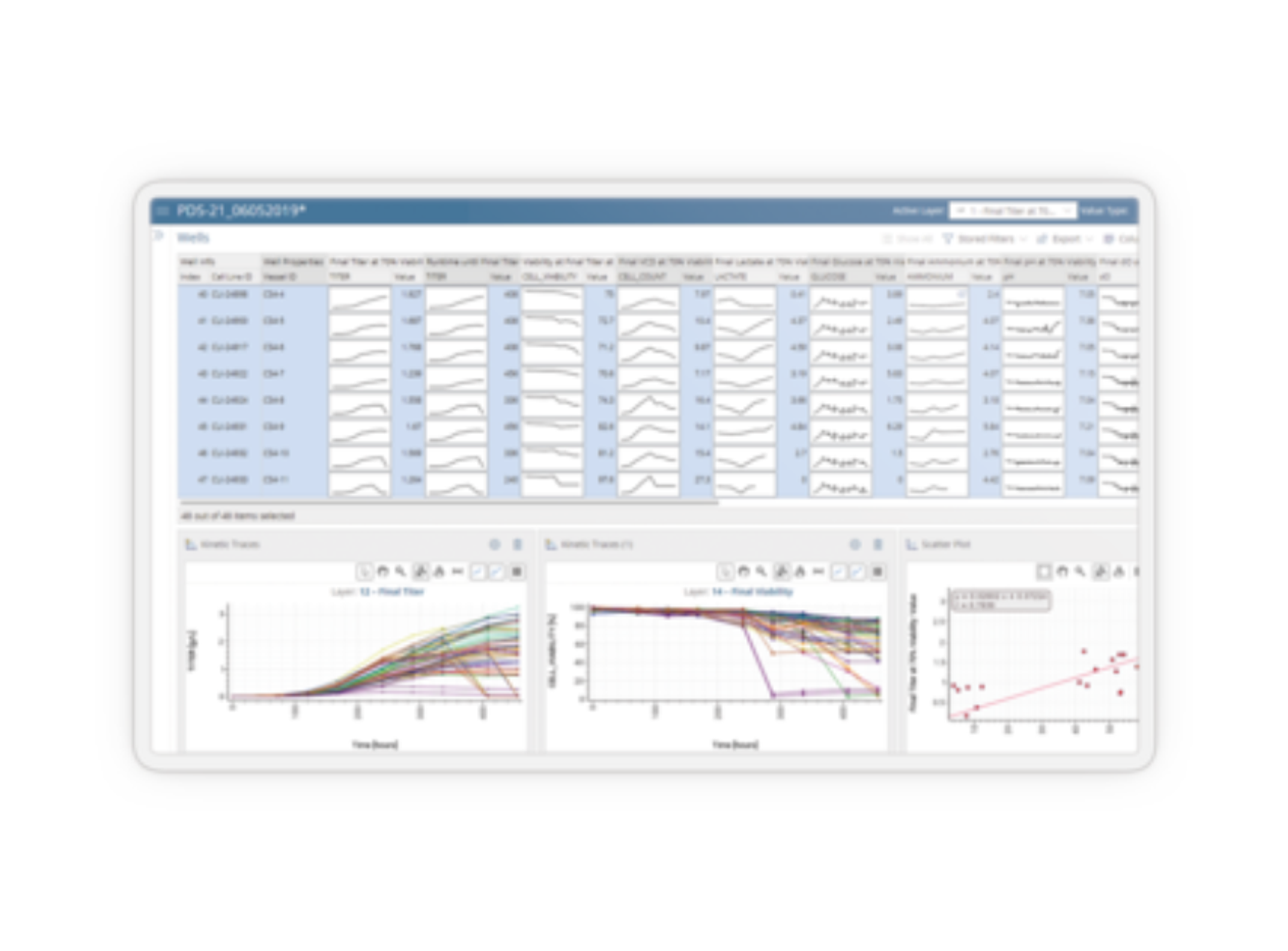
Analytical E-Lab Notebook
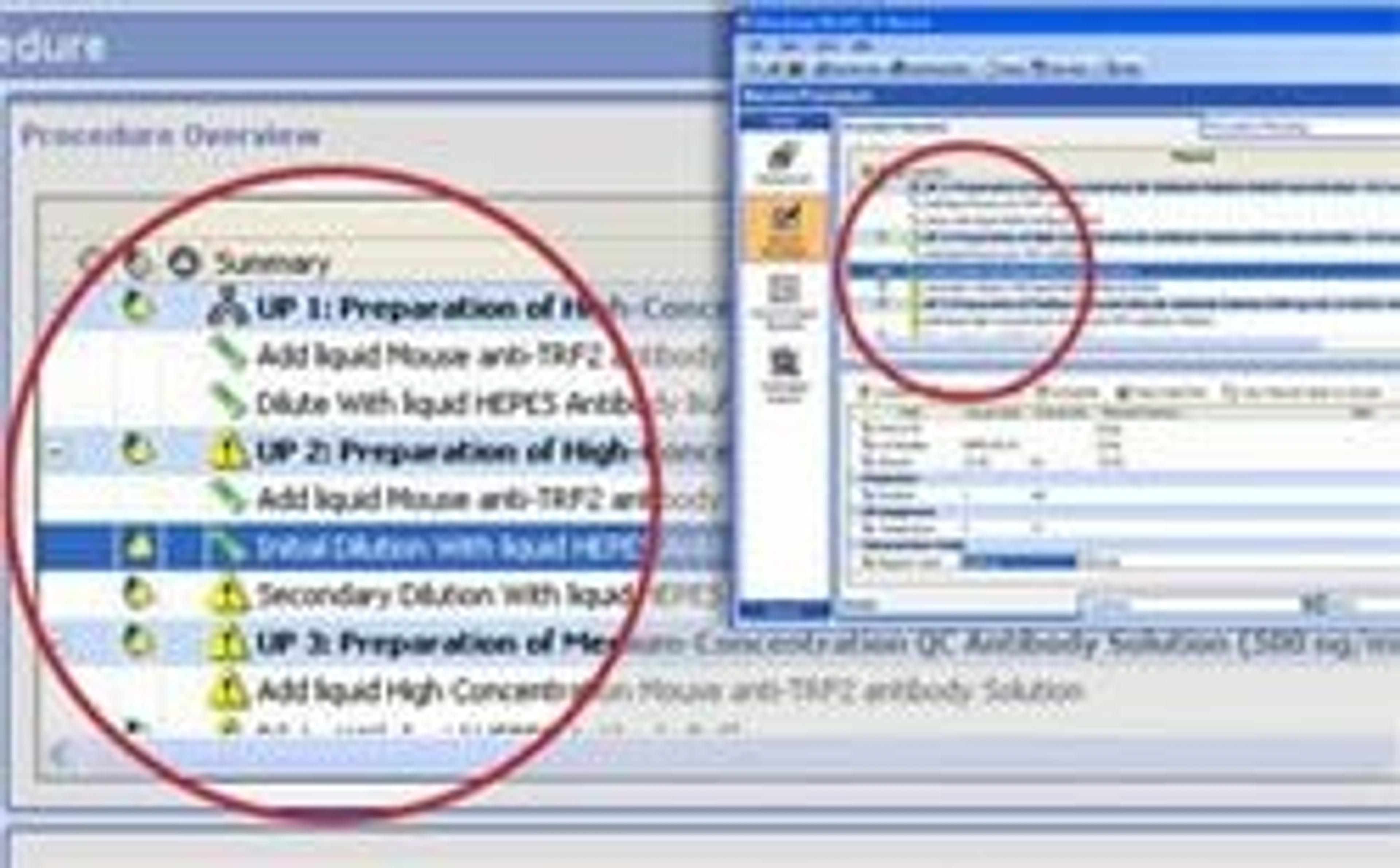
Symyx Software Analytical Notebook is an Electronic Laboratory Notebook (ELN) specifically designed to enable analytical chemists to use prior experience, develop new methods, access relevant information, and integrate to critical systems to be more efficient and effective. Prepare: Design experiments and data templates Flexible data entry tools allow scientists to plan all types of experiments, including method developme…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Symyx Software Analytical Notebook is an Electronic Laboratory Notebook (ELN) specifically designed to enable analytical chemists to use prior experience, develop new methods, access relevant information, and integrate to critical systems to be more efficient and effective.
Prepare: Design experiments and data templates
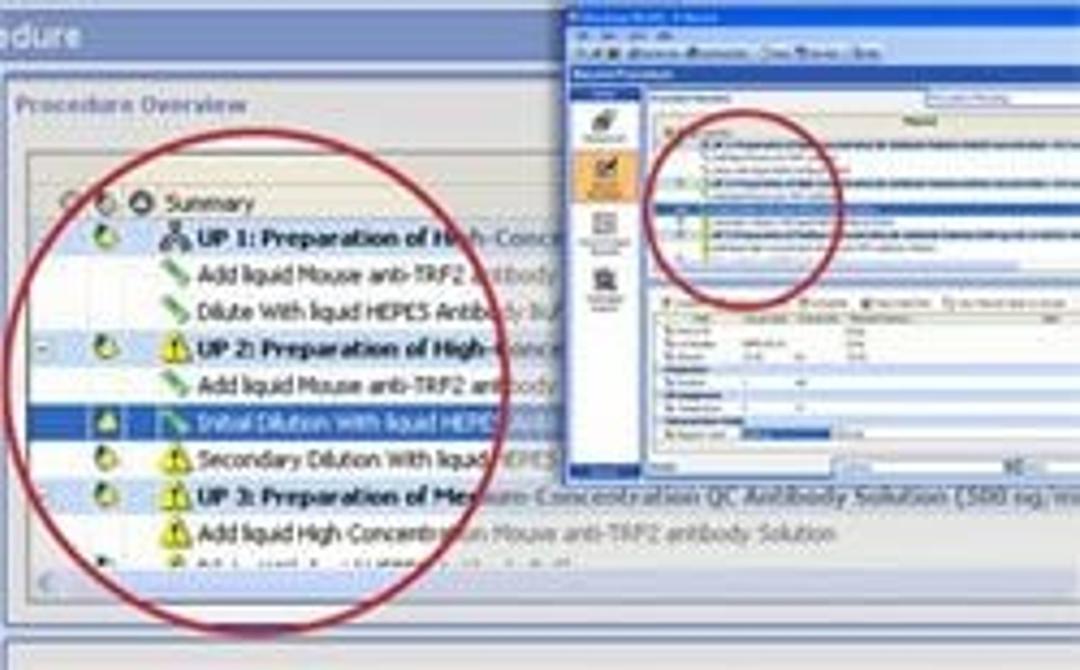
- Flexible data entry tools allow scientists to plan all types of experiments, including method development, routine analysis, and ad hoc experimentation.
- Creating templates for experimental data using our automated tools saves time, increases template consistency, thereby reducing mistakes.
- Finding past experiments in a company-wide repository allows scientists to access corporate knowledge and design better experiments.
Document: Record all types of analytical experiments
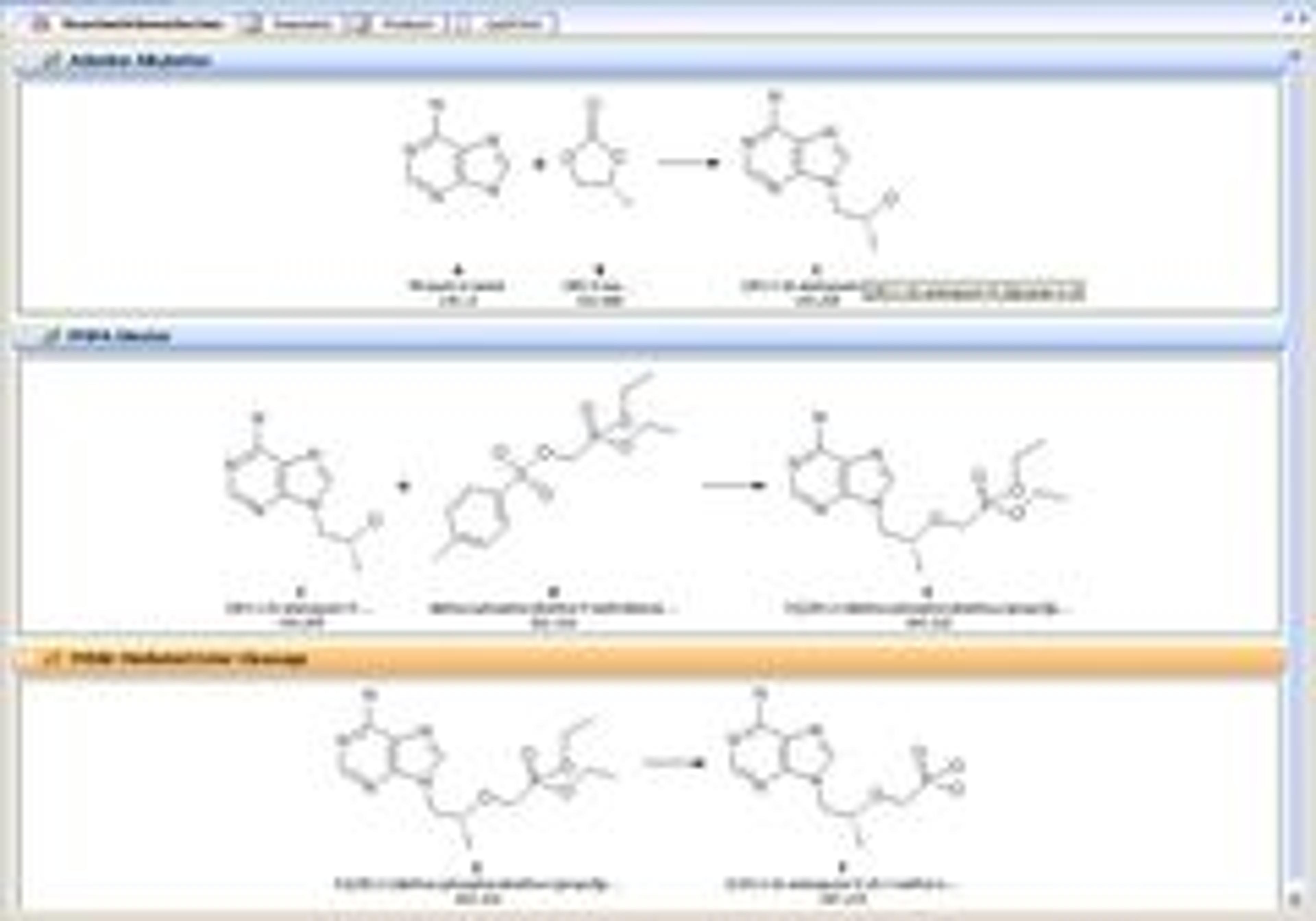
- Recording routine analyses in templates saves time, reduces mistakes, and eases the compliance burden.
- Recording method development in a searchable repository improves method quality and saves time by allowing scientists to easily find and reuse past methods.
- Recording ad hoc experimentation in a company-wide repository simplifies data transfer and allows scientists to easily learn from corporate knowledge.
Analyze: Rapidly find experimental data
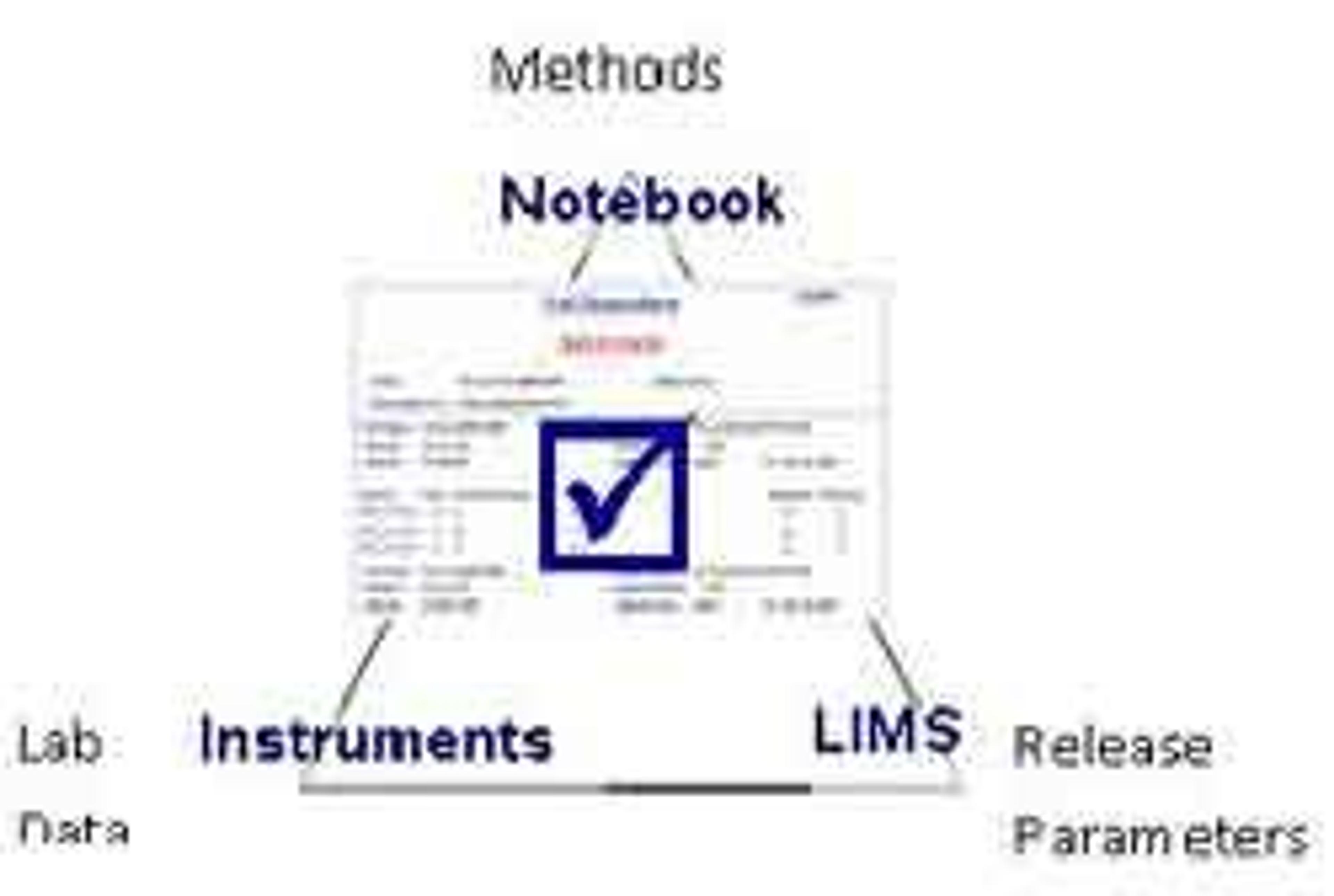
- A searchable global data repository allows users to rapidly find experimental data using searches such as project, structure, and investigator.
- A single Notebook platform spanning discovery and development, facilitates rapid data aggregation for reports, including FDA submissions.
- Audit trails allow users to track the history of documents, simplifying compliance.