BioPharma Solution
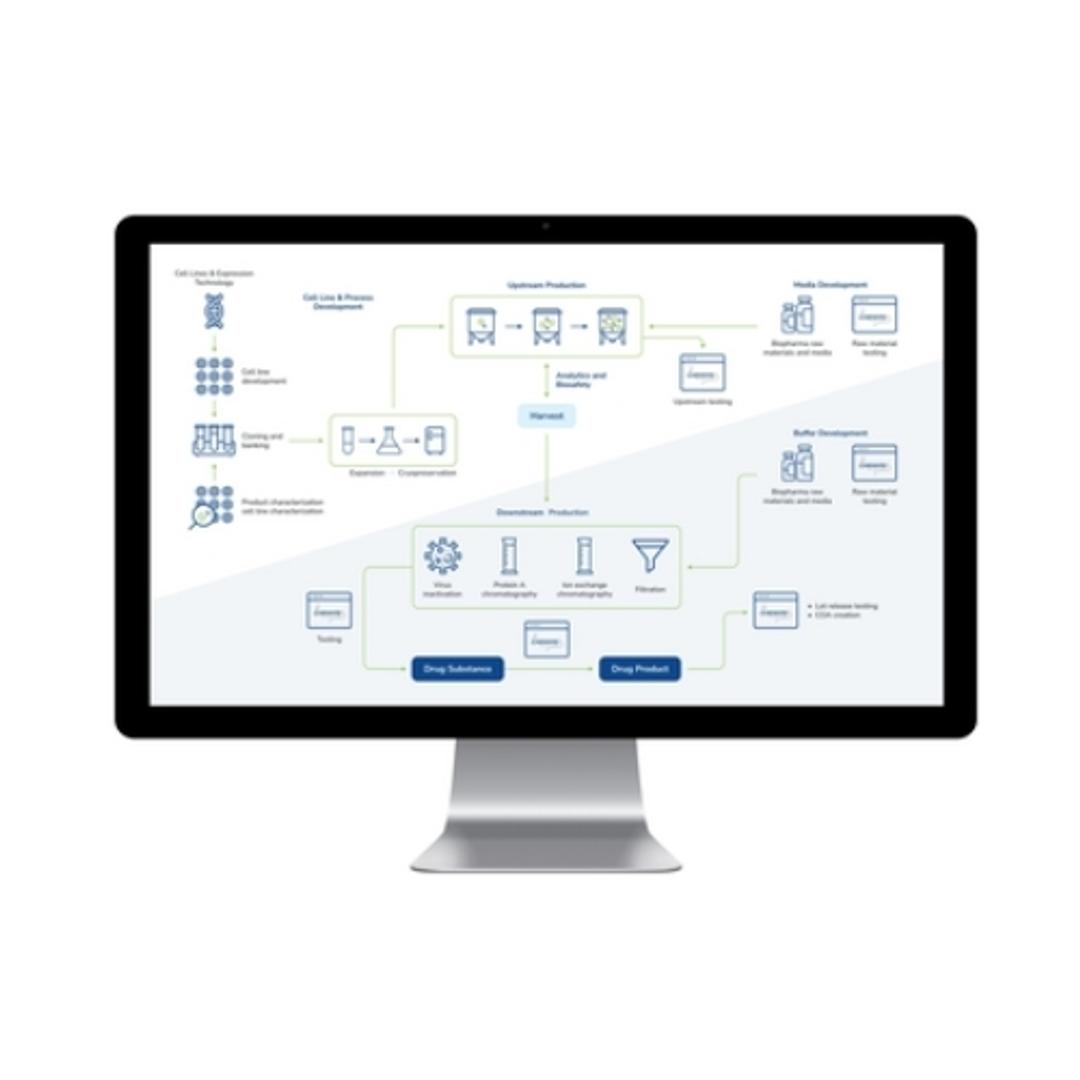
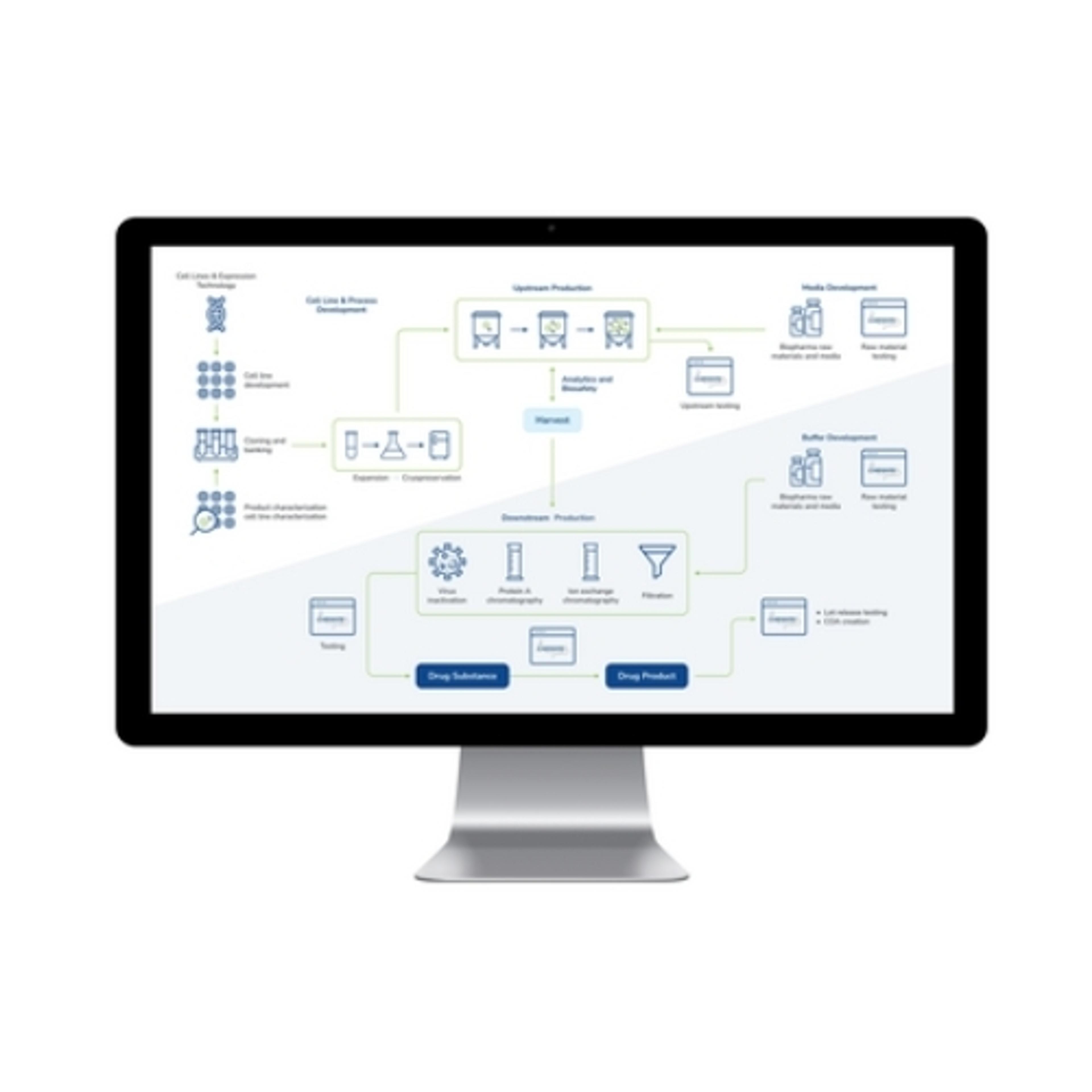
LabWare’s BioPharma Solution is a leading laboratory software platform designed for biopharmaceutical and biotechnology industries, promoting quality throughout the development and production lifecycle. The LabWare Laboratory Information Management System (LIMS) platform streamlines complex processes, from cell line development and banking to the intricate steps of biopharmaceutical manufacturing, including upstream and downst…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
LabWare’s BioPharma Solution is a leading laboratory software platform designed for biopharmaceutical and biotechnology industries, promoting quality throughout the development and production lifecycle. The LabWare Laboratory Information Management System (LIMS) platform streamlines complex processes, from cell line development and banking to the intricate steps of biopharmaceutical manufacturing, including upstream and downstream processing. With its emphasis on automation, real-time data tracking, and stringent compliance enforcement, the LabWare BioPharma Solution is a good choice for laboratories seeking efficiency, accuracy, and regulatory adherence in their operations.
LabWare BioPharma Solution key features include:
- Streamlined Biopharmaceutical Processes: Optimizes cell line development, upstream and downstream processing, and media & buffer formulation, promoting adherence to GMP for high-quality products.
- Advanced Instrumentation Support: Facilitates automation and paperless operations through seamless interfacing with laboratory analyzers and robotics, enhanced by a configurable Rules Engine for increased efficiency.
- Regulatory Compliance and Data Integrity: Through robust data management and audit trails, LabWare promotes comprehensive documentation, traceability, and compliance with critical regulations and industry standards, including 21 CFR Part 11 and ISO 17025.
- Operational Efficiency and Insight: Offers real-time tracking, built-in laboratory KPIs, and configurable dashboards for data review and process optimization, alongside comprehensive tools for stability study management and inventory control.