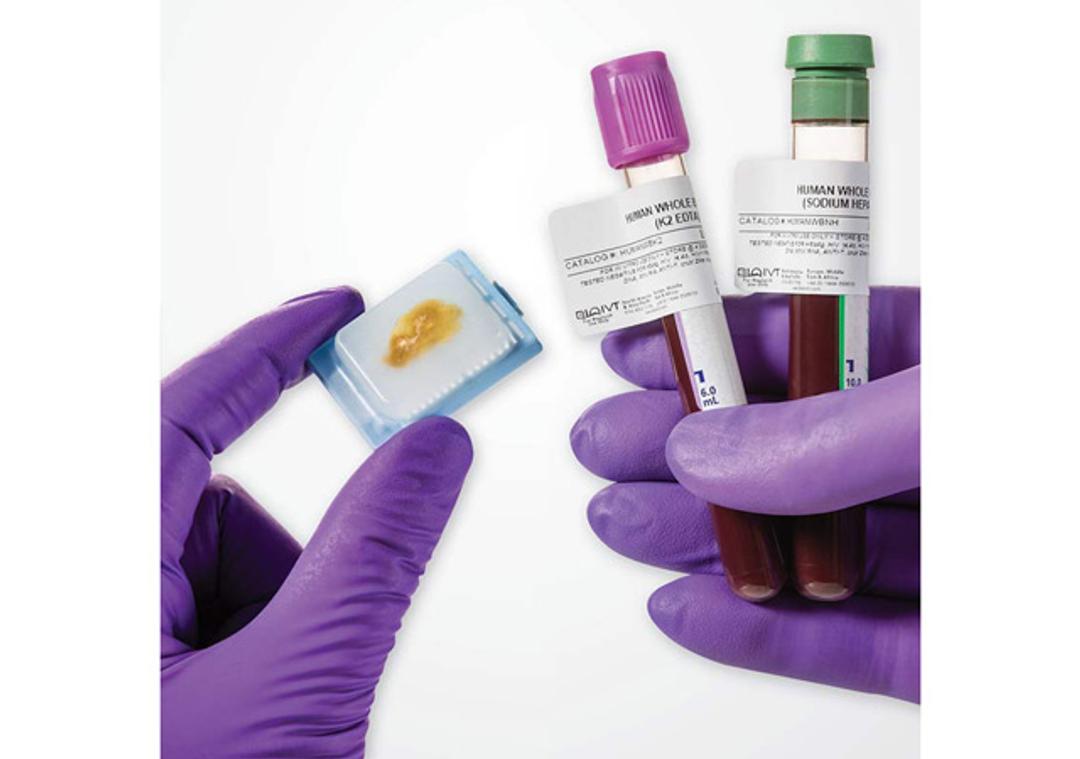
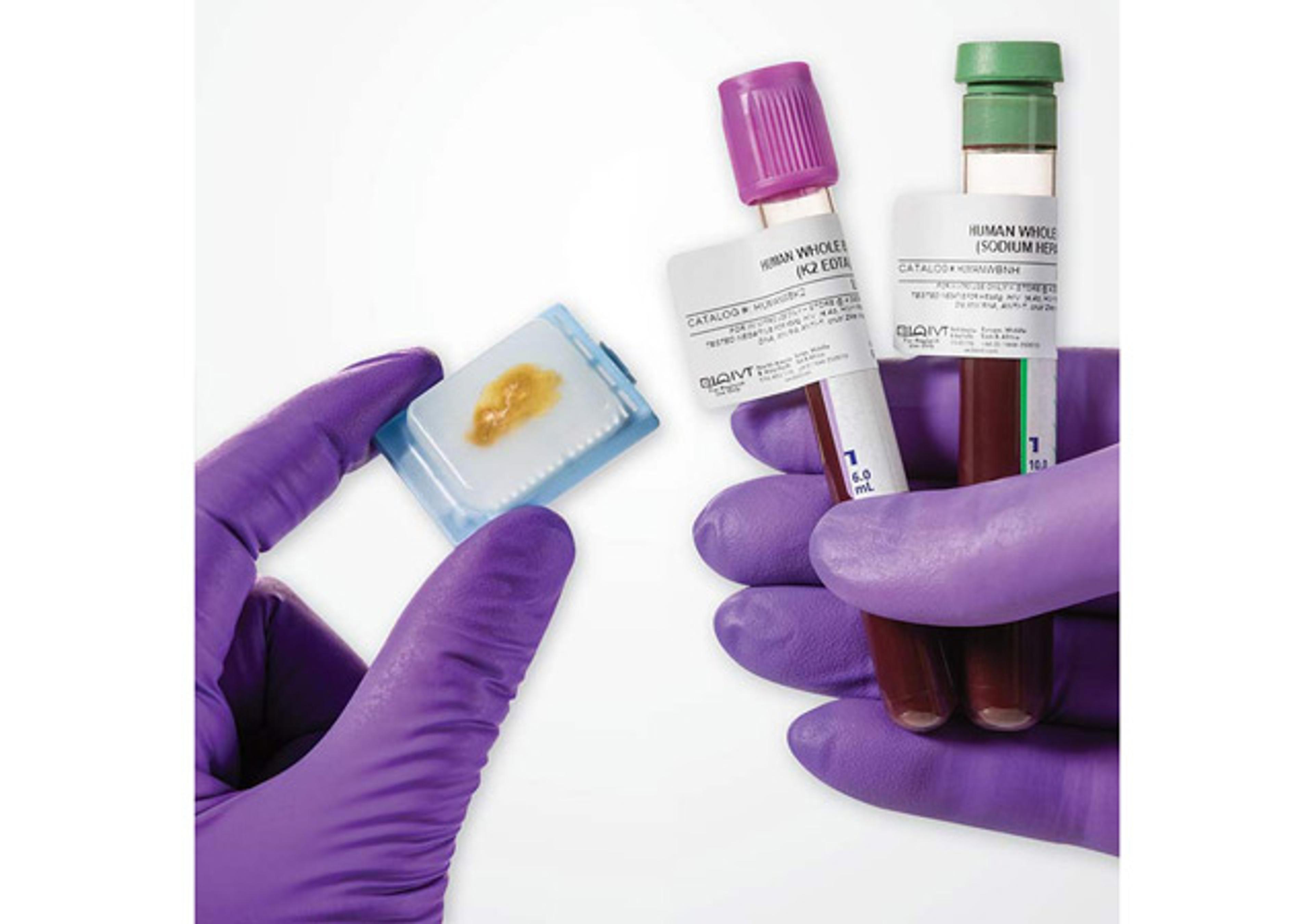
Clinically-Collected Specimens
BioIVT has the expertise in the processes required for ethical acquisition of biospecimens that cover a wide range of disease states and the protocols needed to produce reliable data. With over 425 IRB-approved clinical sites, BioIVT provides clinical specimens for nearly all disease states.
Place your order directly with the manufacturer.
BioIVT has the expertise in the processes required for ethical acquisition of biospecimens that cover a wide range of disease states and the protocols needed to produce reliable data. With over 425 IRB-approved clinical sites, BioIVT provides clinical specimens for nearly all disease states, such as:
- Oncology tissues with board-certified pathology confirmation of diagnosis
- Pre- and post-treatment biofluids
- Infectious disease/serology cohorts with confirmed or suspected diagnosis
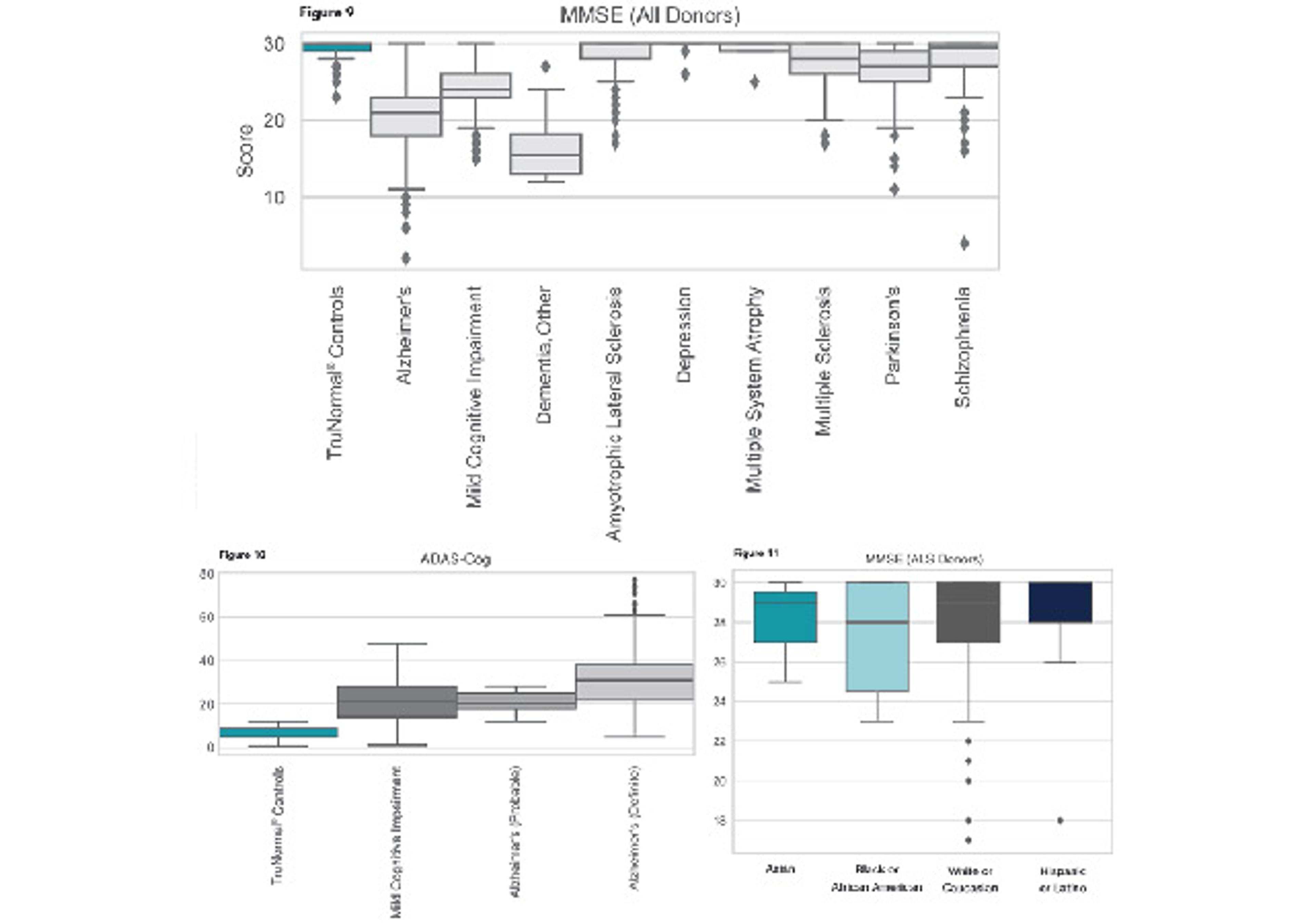
- Clinical remnant samples with associated clinical data and test results representing autoimmune, cardiovascular, dermatological, neurological and endocrine diseases, among others
Mutation analysis is also available with around 10,000 specimens with next-generation sequencing (NGS) data and the capacity to screen 600+ biospecimens per month.