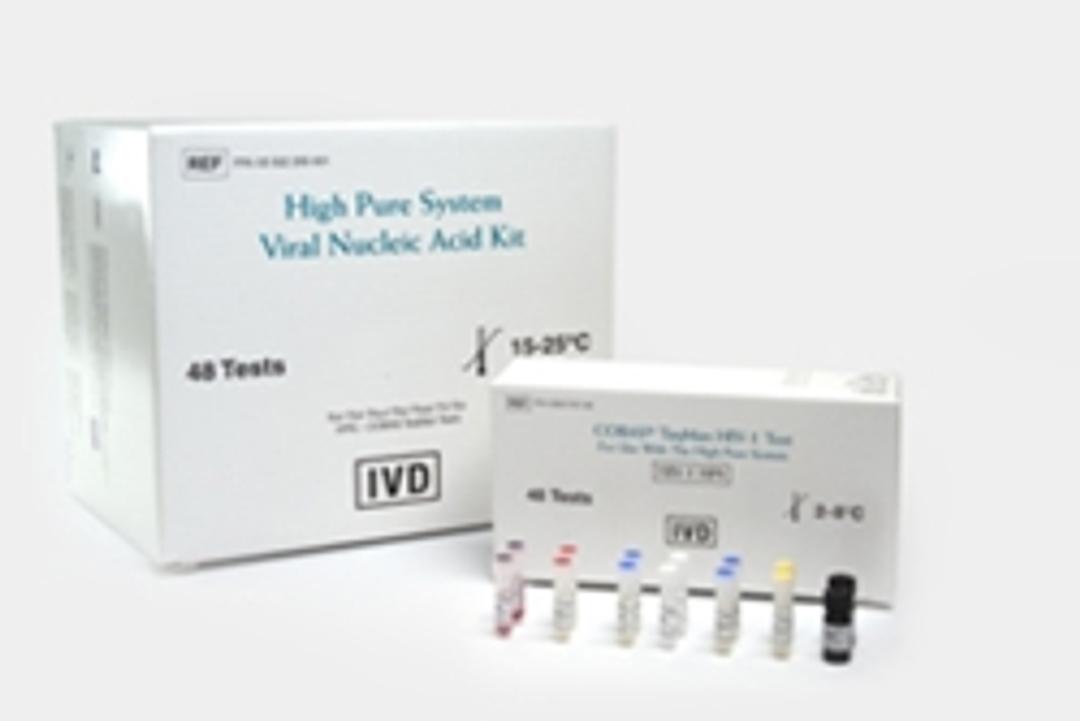
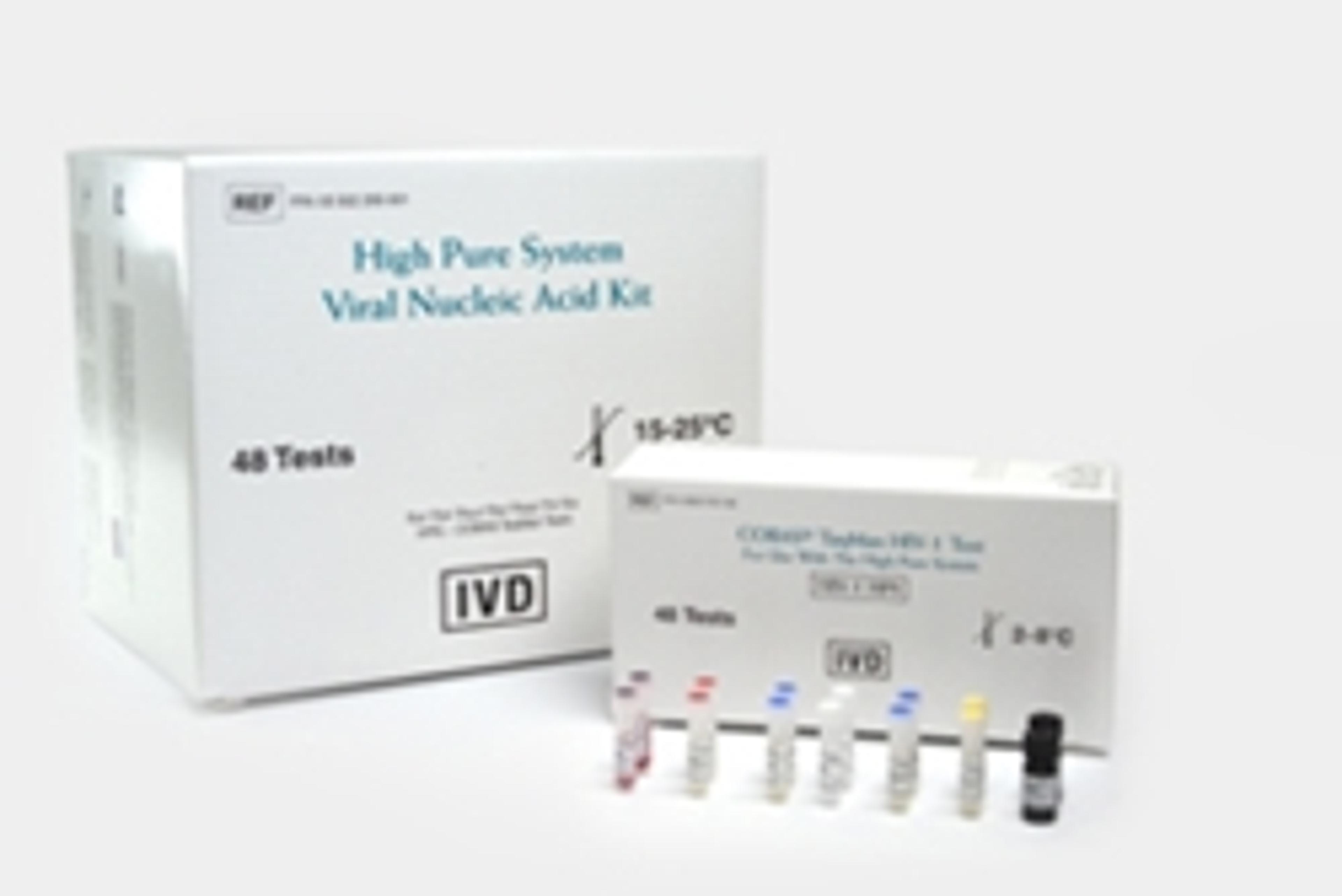
COBAS® TaqMan® HIV-1 Test
Human Immunodeficiency Virus Type 1 (HIV-1) viral load quantification. An in vitro nucleic acid amplification test for the quantitation of HIV-1 RNA in human EDTA plasma.The High Pure System Viral Nucleic Acid Kit allows manual specimen preparation and the COBAS® TaqMan® 48 Analyzer automates amplification and detection.To assess patient prognosis or to monitor the effects of anti-retroviral therapy, the test measures the bas…
Human Immunodeficiency Virus Type 1 (HIV-1) viral load quantification.
An in vitro nucleic acid amplification test for the quantitation of HIV-1 RNA in human EDTA plasma.
The High Pure System Viral Nucleic Acid Kit allows manual specimen preparation and the COBAS® TaqMan® 48 Analyzer automates amplification and detection.
To assess patient prognosis or to monitor the effects of anti-retroviral therapy, the test measures the baseline HIV-1 RNA level by measuring changes in plasma HIV-1 RNA levels during the course of anti-retroviral treatment.
The test is intended to be used in conjunction with clinical presentation and other laboratory markers of disease progress for the clinical management of HIV-1 infected patients.
The COBAS® TaqMan® HIV-1 Test For Use With The High Pure System (CE-IVD) is not intended to be used as a screening test for blood or blood products for the presence of HIV-1 or as a diagnostic test to confirm the presence of HIV-1 infection.
Features & Benefits:
- Delivers highly accurate results faster – an important advantage for clinicians
- Highly sensitive and accurate
- Minimizes testing errors with closed-tube amplification and detection on the COBAS® TaqMan® 48 Analyzer
- Improves result integrity – AmpErase enzyme reduces the risk of cross-contamination of samples or labs
- Provides a broader range of viral load data than earlier generation tests