Comet Assay IV - scoring system
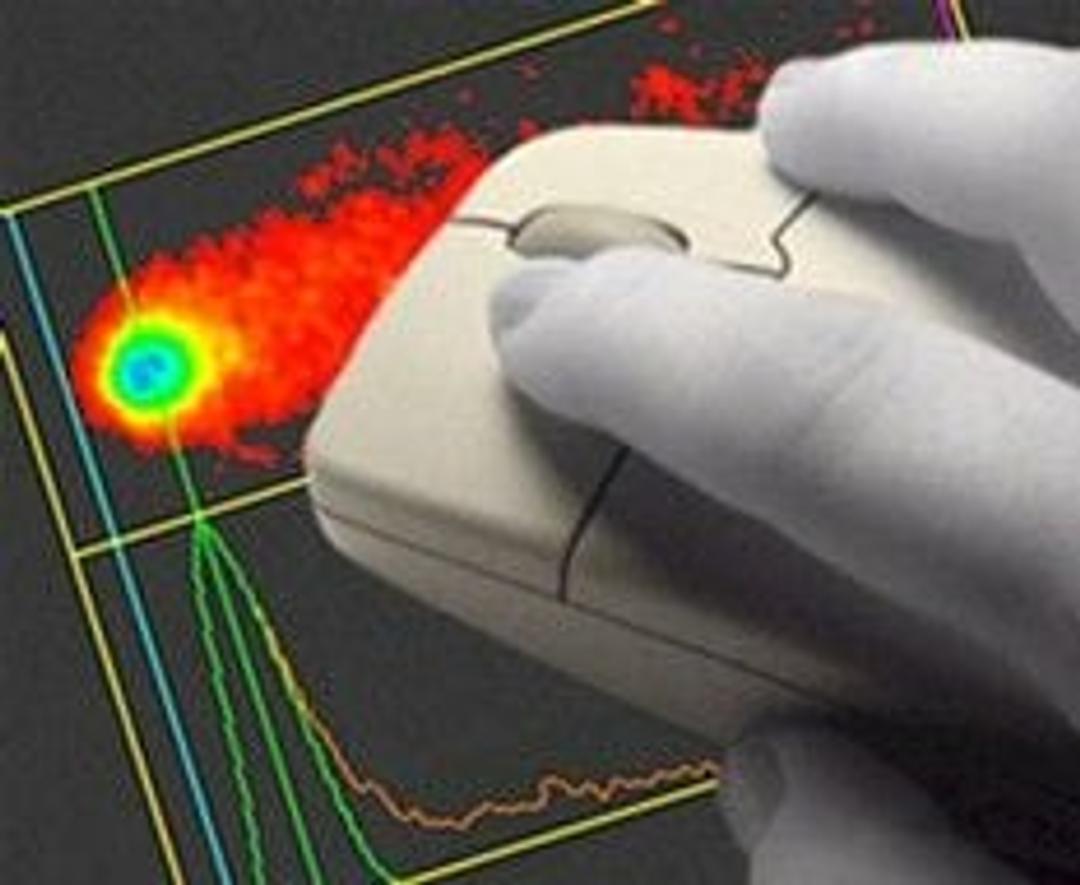
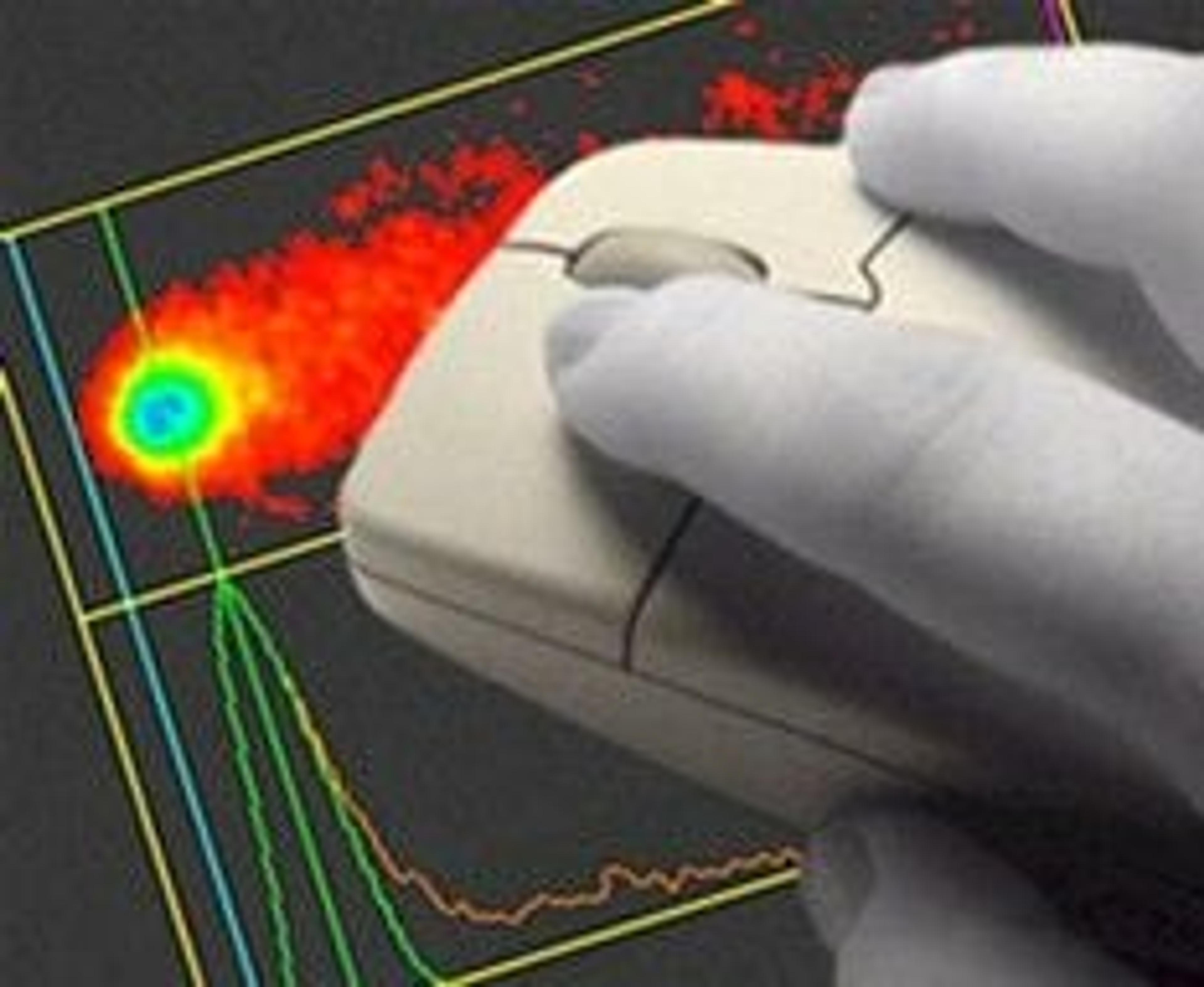
Comet Assay IV's unique single-click scoring method and ultra-fast live video picture make it the most efficient and easy-to-use system available for measuring DNA damage using single cell gel electrophoresis. There are no complicated parameters to adjust and no hardware to install - simply connect the camera and start scoring. Working closely with leading experts in the field, Perceptive Instruments have released Comet Assa…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Comet Assay IV's unique single-click scoring method and ultra-fast live video picture make it the most efficient and easy-to-use system available for measuring DNA damage using single cell gel electrophoresis. There are no complicated parameters to adjust and no hardware to install - simply connect the camera and start scoring.
Working closely with leading experts in the field, Perceptive Instruments have released Comet Assay IV - the very latest in a highly successful series of systems that have developed and evolved over the past twelve years. Fully validated and featuring comprehensive audit trails, Oracle database connectivity and entry of reasons for edits, deletes etc., Comet Assay IV is the professional choice for those seeking compliance with GLP and FDA 21 CFR part 11.
- Unique single click automatic scoring
for unrivalled speed and reproducibility - Flexible study management facilities
to help you work your own way - Meet regulatory requirements
with Comet Assay IV’s comprehensive compliance options - Oracle database connectivity
for optimal security of result and audit data - Powerful Excel macros
for analysis & comparison of data - Automatically saves images
with data for Quality Assurance audit - User account management options
for GLP and FDA 21 CFR Part 11 compliance - Comprehensively tested and validated
in accordance with our stringent ISO 9001:2000 and TickIT approved testing procedures