COVID-19 ImmunoRank™ Neutralization MICRO-ELISA Kit
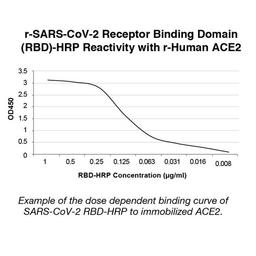
The COVID-19 ImmunoRank™ Neutralization MICRO-ELISA test for detection of circulating SARS-CoV-2 neutralizing antibodies. The assay is designed to detect antibodies of all Ig classes in human plasma or serum that bind to the SARS-CoV-2 receptor binding domain (RBD) and are capable of blocking the binding of the RBD to angiotensin-converting enzyme 2 (ACE2), the viral entry receptor on the surface of target cells.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
The COVID-19 ImmunoRank™ Neutralization MICRO-ELISA is a test for semi-quantitative detection of SARS-CoV-2 neutralizing antibodies in plasma or serum. This assay is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2 indicating recent or prior infection and detection of circulating SARS-CoV-2 neutralizing antibodies of all Ig classes. At this time, it is unknown for how long antibodies persist following infection and if the presence of neutralizing antibodies confers protective immunity. The COVID-19 ImmunoRank™ Neutralization MICRO-ELISA test should not be used to diagnose acute SARS-CoV-2 infection.
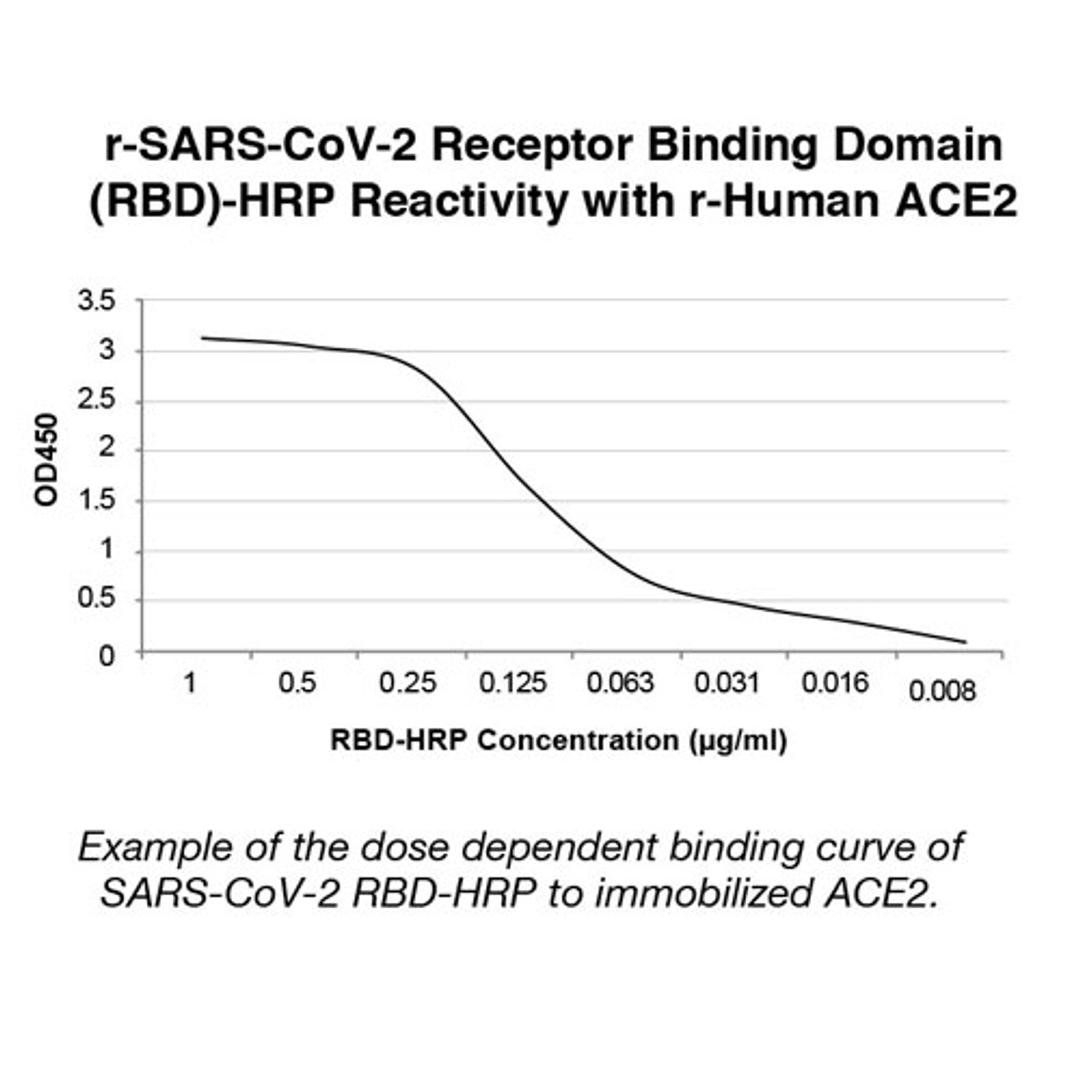
The test is a solid phase enzyme-linked immunosorbent assay (ELISA) using a chromogenic enzyme substrate as an indicator. The SARS CoV-2 recombinant viral entry receptor protein, angiotensin-converting enzyme 2 (ACE2), is immobilized to polystyrene wells of a microplate (solid phase). In the wells of a separate incubation plate, a positive control, negative control, calibrator control and test specimens are diluted and added to the wells along with the diluted soluble recombinant SARS CoV-2 receptor binding domain (RBD) recombinant protein conjugated to horseradish peroxidase.
Features
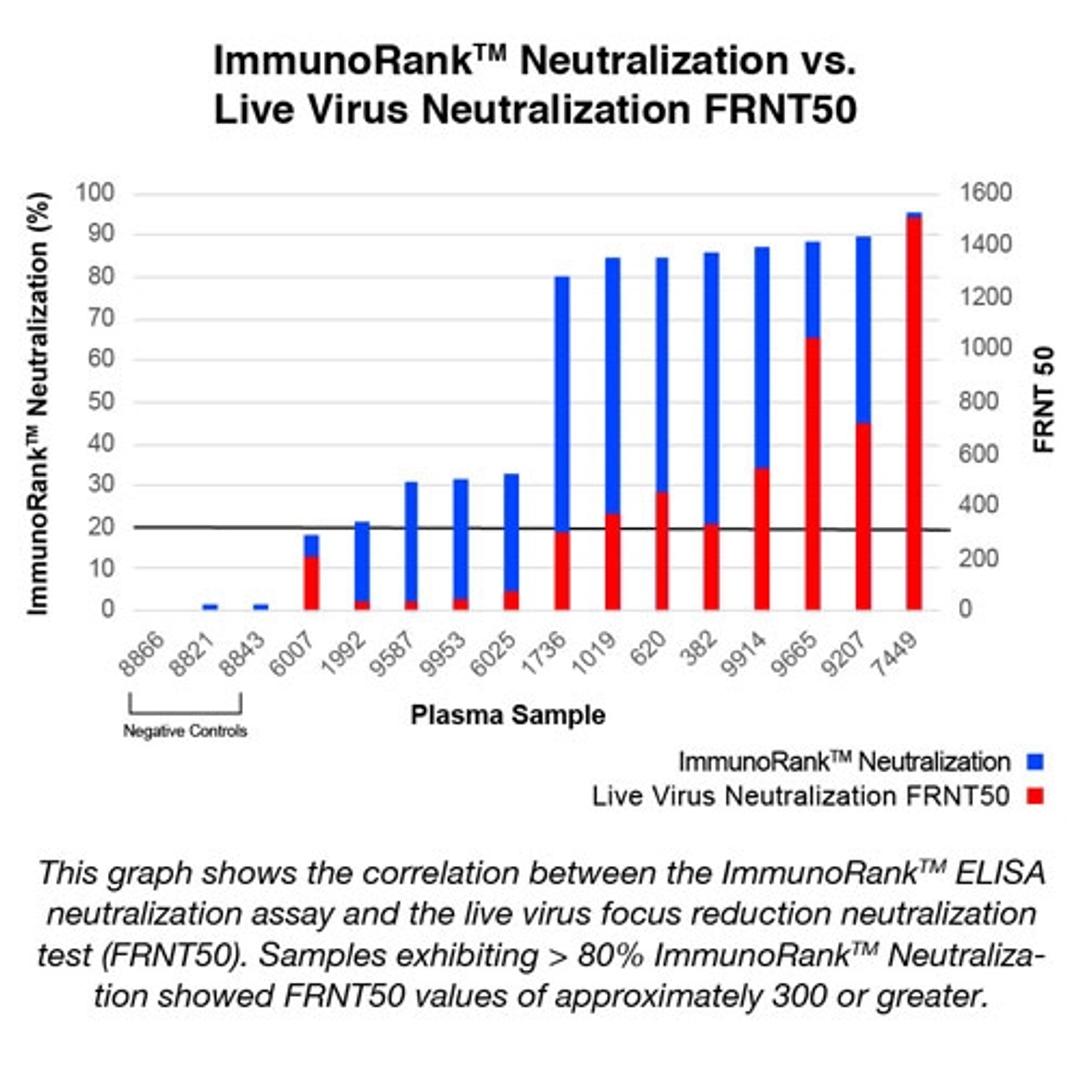
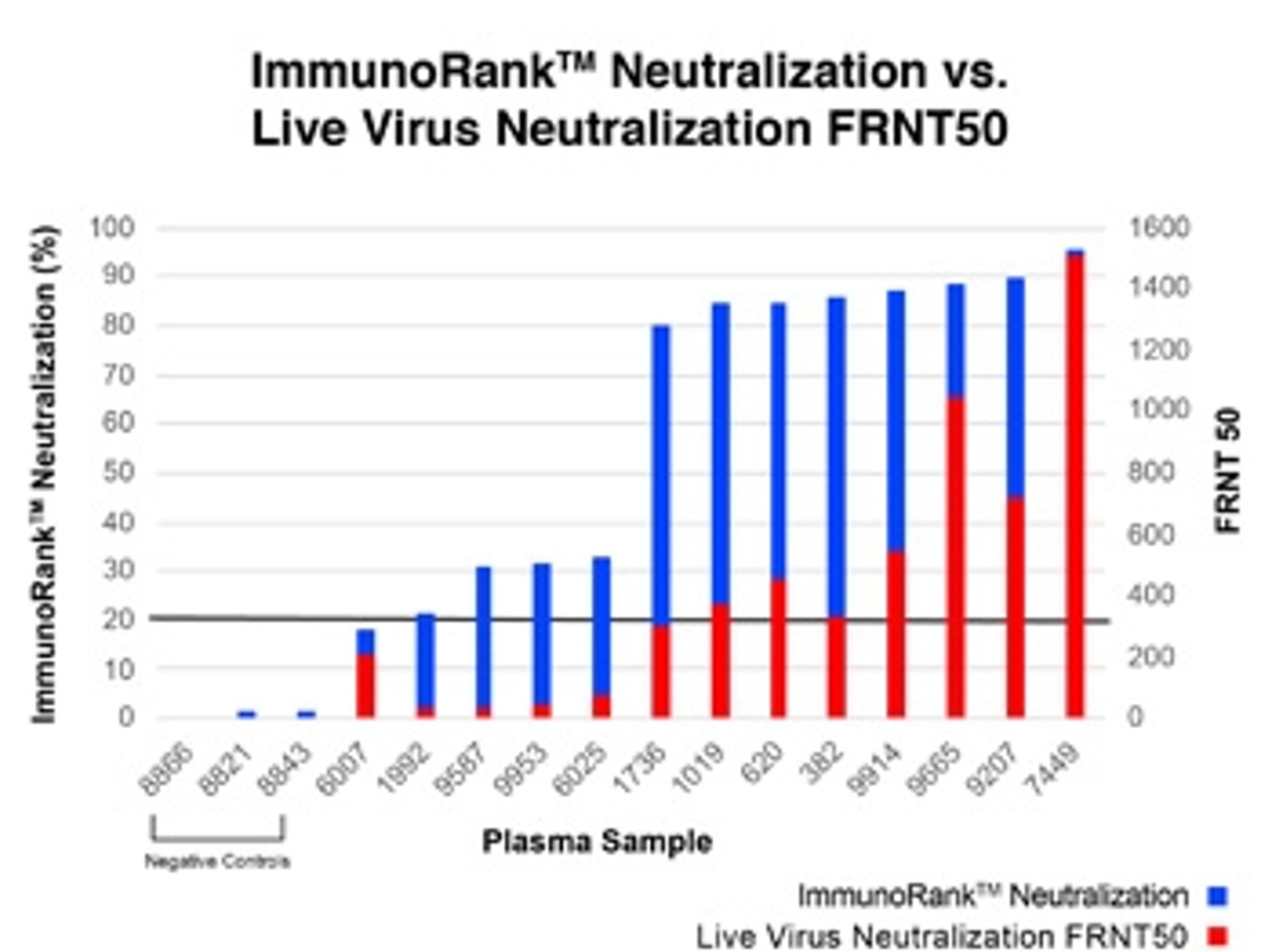
- ImmunoRank™ offers a faster, simpler, more cost effective way to identify high titer convalescent plasma for use in both treating COVID-19 patients and for creating COVID-19 hyperimmune globulins
- ImmunoRank™ can test up to 90 samples per test kit with 99.8% specificity.
- Semi-quantitative assay range of 0-100% neutralization
Applications
- ELISA Det