Darwin
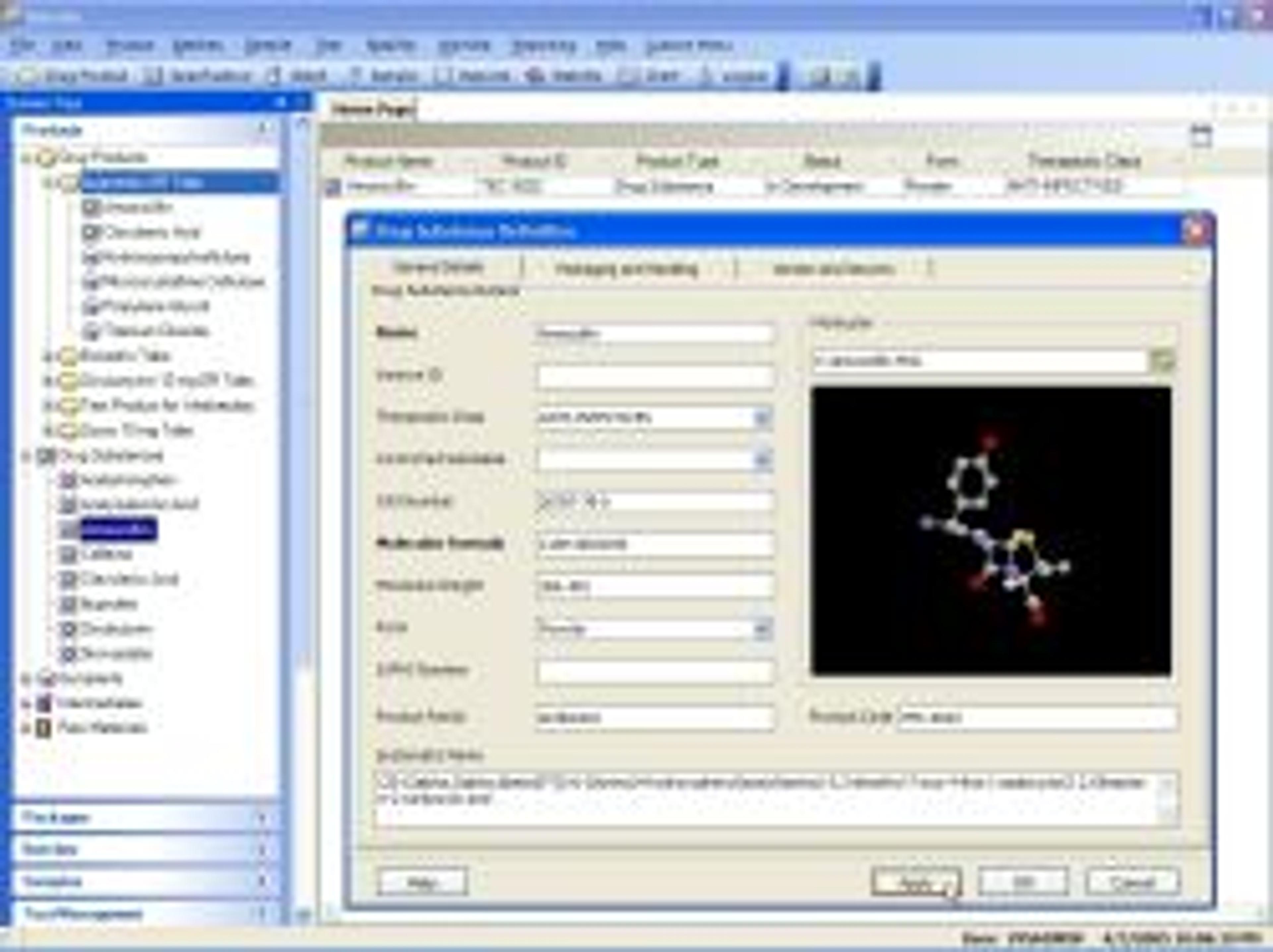
Darwin LIMS is specifically designed to address the unique needs of pharmaceutical manufacturing R&D and QA/QC labs. Darwin's unique batch and product oriented design aligns directly with pharmaceutical manufacturing processes, allowing R&D and production data to be logically organized, summarized and reported. By including industry specific standard functionality that is directly supported by Thermo's helpdesk, Darwin provide…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Darwin LIMS is specifically designed to address the unique needs of pharmaceutical manufacturing R&D and QA/QC labs. Darwin's unique batch and product oriented design aligns directly with pharmaceutical manufacturing processes, allowing R&D and production data to be logically organized, summarized and reported. By including industry specific standard functionality that is directly supported by Thermo's helpdesk, Darwin provides – out of the box – nearly everything pharmaceutical manufacturing labs need, and nothing they don't.
The unpredictable costs and risks associated with customizing a traditional Laboratory Information Management System to serve the needs of pharmaceutical manufacturing are giving rise to the trend toward application-specific, commercial off-the-shelf (COTS) software solutions. Darwin LIMS meets pharmaceutical-specific requirements as a standard commercial solution. Among Darwin’s industry-specific functionality, which are covered in the user manual and supported by the helpdesk, the system handles:
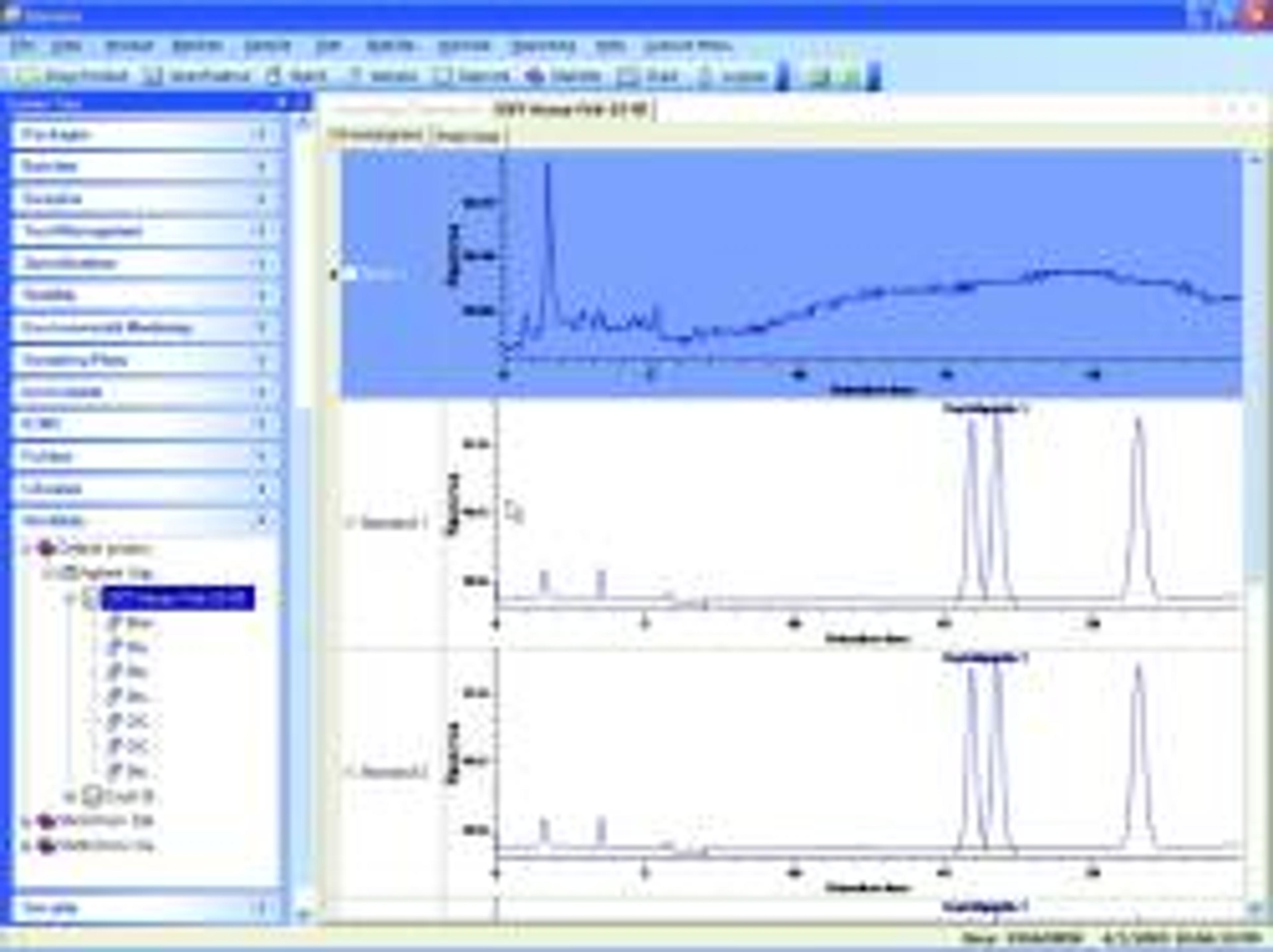
- Dissolution (Single-point and Multi-point)
- Content Uniformity
- Stability Management
- Product Management
- Batch Management
- System Interfacing
Pharmaceutical Manufacturing R&D
Because Darwin can be used by both R&D and QC, there is no need to manually transfer product R&D to QC. Darwin’s batch- and product-orientation allow R&D data to be more easily stored, summarized and reported than sample-only systems. And while meeting the rigid compliance requirements of QC, Darwin regulates compliance requirements based on the type of data being manipulated – enabling a more flexible approach for R&D while within the same application.
Pharmaceutical QA/QC
In addition to providing complete testing solutions within the core feature set, Darwin embeds standard business logic to accelerate decision-making and incorporates notification tools to improve communications. The solution’s stability management tools accommodate a variety of study designs, and study data can be quickly summarized for reporting. Darwin also tracks batch hierarchy and genealogy, enabling batch/lot release testing results to be associated with final product.
Business benefits
Lowering total cost of ownership is more easily achieved with Darwin, when used to standardize company processes. A user-friendly and intuitive interface ensures end-user acceptance and support for process modification as part of standardization. Lab managers can maximize use of common functionality and eliminate or reduce customization by deploying Darwin. In the instances where customization is necessary, Darwin uses standard programming tools like Microsoft® Visual Studio .NET, not proprietary programming or scripting languages.
Darwin is designed to:
- Support the product entity – batches and specifications are tied to correct product
- Handle complex study designs – supporting and shortening time to define protocols
- Track inventory, provide notification – reducing risk of protocol deviations
- Easily filter, sort and report data – users rapidly summarize and report data without IT intervention
- Embed multi-stage test logic – for complex aggregate tests like dissolution and content uniformity
- Enable standardization – solid foundation for multi-site harmonization
- Lower total cost of ownership while improving productivity and end-user experience