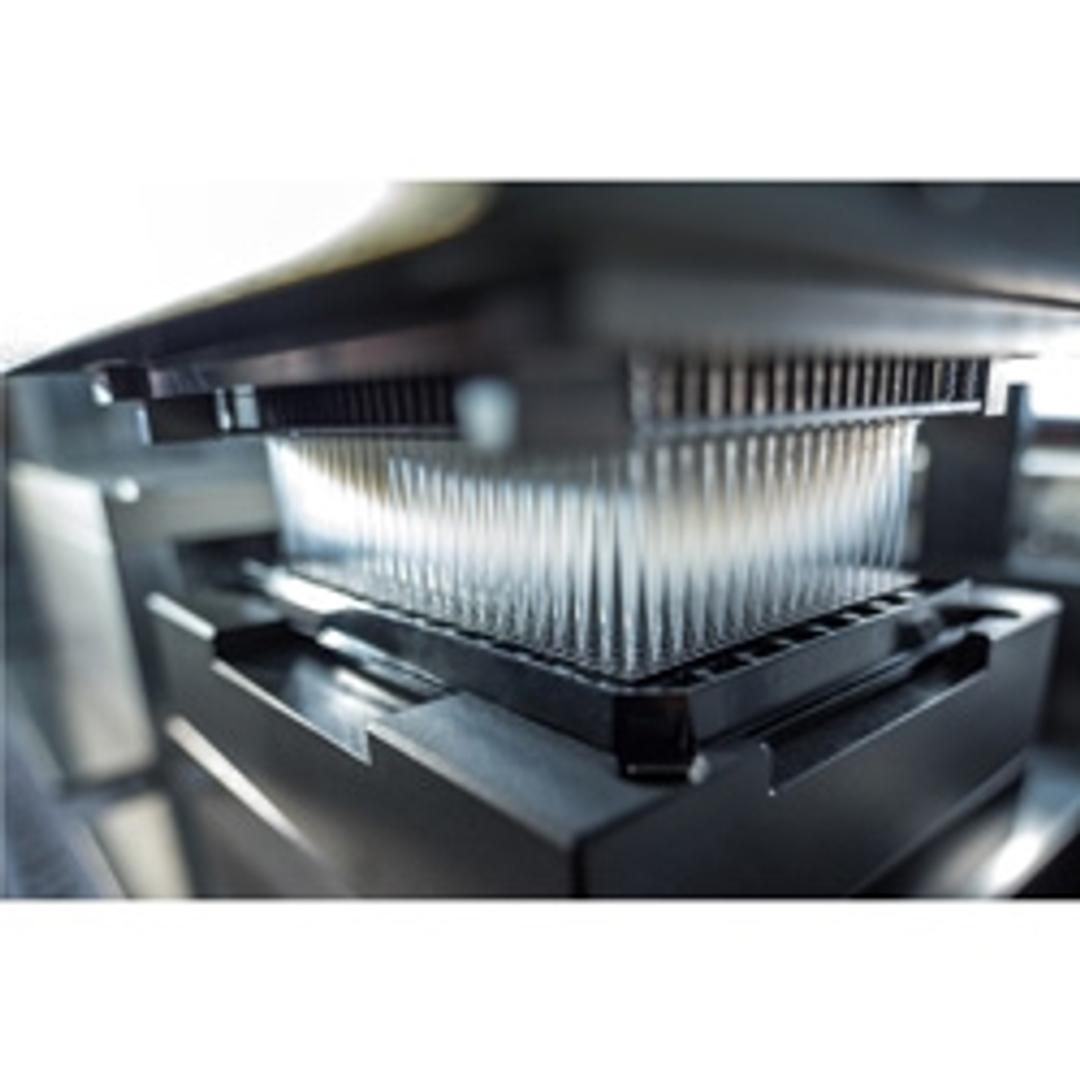
Fluent® Gx Automation Workstation
The Fluent Gx Automation Workstation is designed specifically to meet the needs of clinical and regulated laboratories, with all the advanced process security features required for applications in clinical, GMP and QC facilities.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
The Fluent Gx combines the liquid handling and workflow benefits already familiar to Fluent users – high on-deck capacities, fast processing and intuitive operation – with the Fluent Gx Assurance software, a suite of software features needed to comply with stringent regulatory requirements, including FDA 21 CFR Part 11. Available in three deck sizes offering capacities from 30 to 72 microplates, it features a modular Dynamic Deck™ with a multilevel design that allows flexible integration of complementary devices, and task-specific arms that can be operated simultaneously to ensure timely completion of assays. High definition pipetting using predefined liquid classes ensures optimal accuracy and reproducibility, and the patented Path Finder™ technology automatically programs the fastest, most efficient route for every arm movement, avoiding the need for laborious manual programming.
Fluent Gx’s Flexible Channel Arm™ provides unrivalled flexibility to suit the needs of your laboratory workflow, with a choice of both air- and liquid-displacement pipetting technologies. Productivity can be further increased with dual Flexible Channel Arms, using both pipetting technologies on 16 separate pipetting channels. This is complemented by advanced liquid level sensing using Adaptive Signal Technology™, allowing smaller sample volumes and minimizing the risk of errors, as well as providing exceptional automated error handling. The Robotic Gripper Arm™ smoothly and rapidly delivers labware – including tubes and plates – to the instrument’s pipetting arms and integrated devices without interruption, and the unique Finger Exchange System™ head automatically changes between tubes or plates and vertical or eccentric plate handling.
The Fluent Gx is exceptionally easy to use, with a built-in touchscreen interface that guides users through daily tasks for fast, consistent operation. The Active Stop and Resume function enables the operator to instantly access the deck during a run to correct set-up errors or omissions simply by opening the door. This is complemented by Method Recovery features that allow process runs to be reinstated even following events such as a power cut or computer crash. The platform’s intuitive FluentControl™ software simplifies even the most challenging applications, offering ‘drag and drop’ interfaces, 3D graphics, and Smart Commands to distribute reagents or transfer samples, as well as seamless management of integrated devices to ensure true walkaway operation.
Enhanced process security is essential for highly regulated environments. The Fluent Gx Assurance software – a suite of software features needed to comply with stringent regulatory requirements, including FDA 21 CFR Part 11 – is designed to simplify compliance for regulated facilities. Multi-level user management allows bespoke control of access to protocols and software features. With three default user levels – operator, application specialist and administrator – plus the ability to define custom roles, this is ideal for busy, multi-user labs. For optimal process security without compromising method development, the optional method approval feature ensures that only approved and validated protocols are used for day-to-day activities, while providing application specialists with the ability to create and test new automation workflows. Administration is further simplified for labs using Windows® Active Directory, allowing each employee’s Windows ID and password to provide direct, traceable access to FluentControl.*
Traceability is crucial, and FluentControl’s sample tracking add-on traces every sample, from initial identification using a handheld or integrated 1D/2D barcode scanner through to reporting of final results, including data from Magellan™ data reduction software. A detailed, secure and audit-ready log of all actions for each sample is generated, which can be automatically uploaded to your LIMS. This is complemented by electronic records that provide protected copies of scripts, methods, carrier and labware definitions, liquid classes, and system configurations. Each file has a checksum to guarantee data integrity, and an optional electronic signatures function provides a detailed record – who, when and what, plus the option to explain why – of any changes made. For maximum process security, this feature can also be used to handle processing errors, requiring the user to approve any run that has been completed with errors or warnings.
The Fluent Gx Assurance software also offers a number of software utilities to simplify system management. The Compliance Checker provides rapid, fully automated verification of the integrity of all executable software components – meeting the needs of GMP facilities and supplementing existing cybersecurity measures – while the Data Audit Tool performs a similar function for electronic records, ensuring data integrity. These advanced features have been developed to streamline regulatory activities, helping laboratories to demonstrate compliance with minimal manual inputs.
*Windows Active Directory log-in coming soon.
The FLUENT Gx is an open automation platform product for general laboratory use. It is intended for routine laboratory tasks, such as general purpose pipetting and general purpose liquid handling and robotic processes. Configuration of options may impact intended use – please consult your local Tecan office.
Windows is a registered trademark of Microsoft Corporation, Washington, USA.
Active Directory is a product of Microsoft Corporation, Washington, USA.