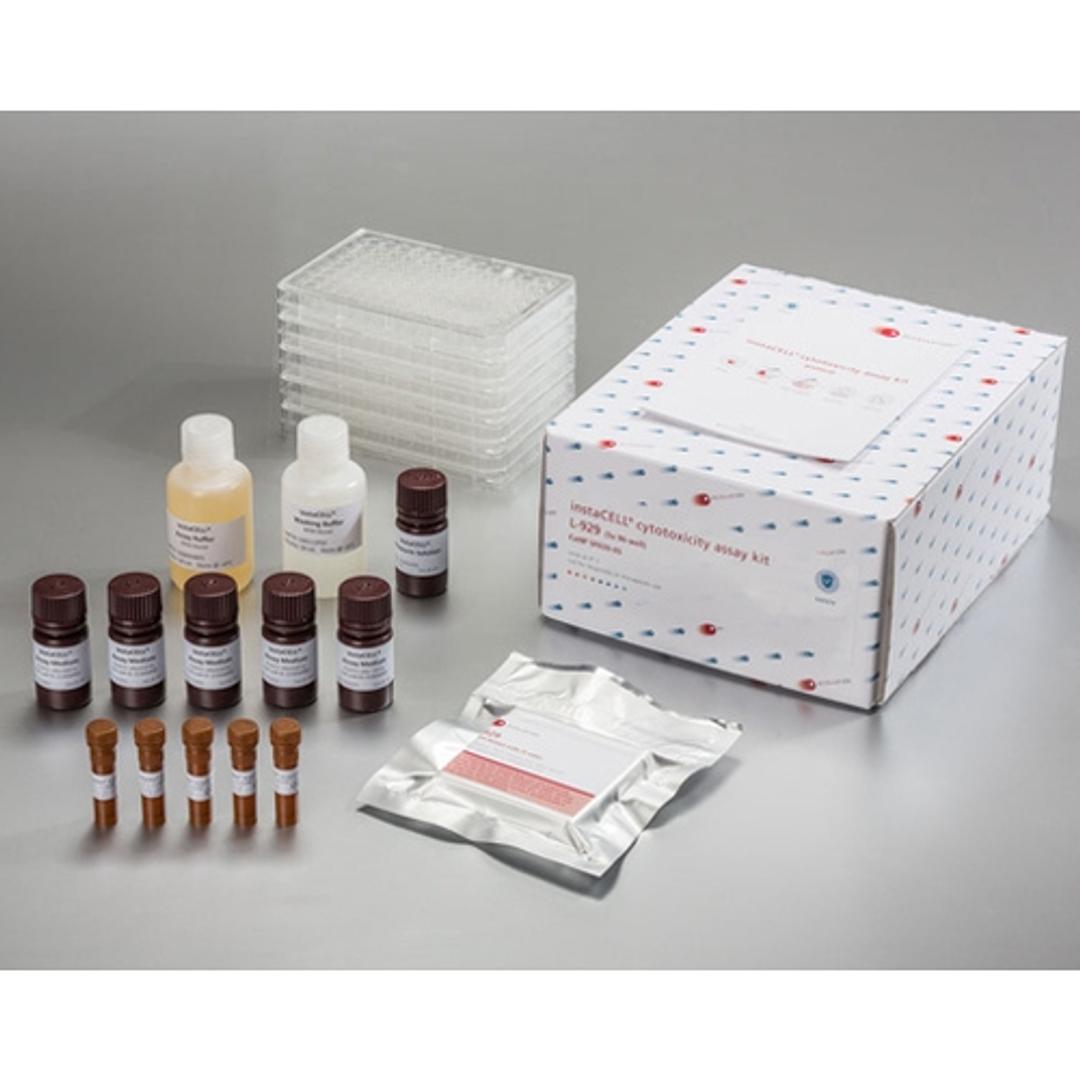
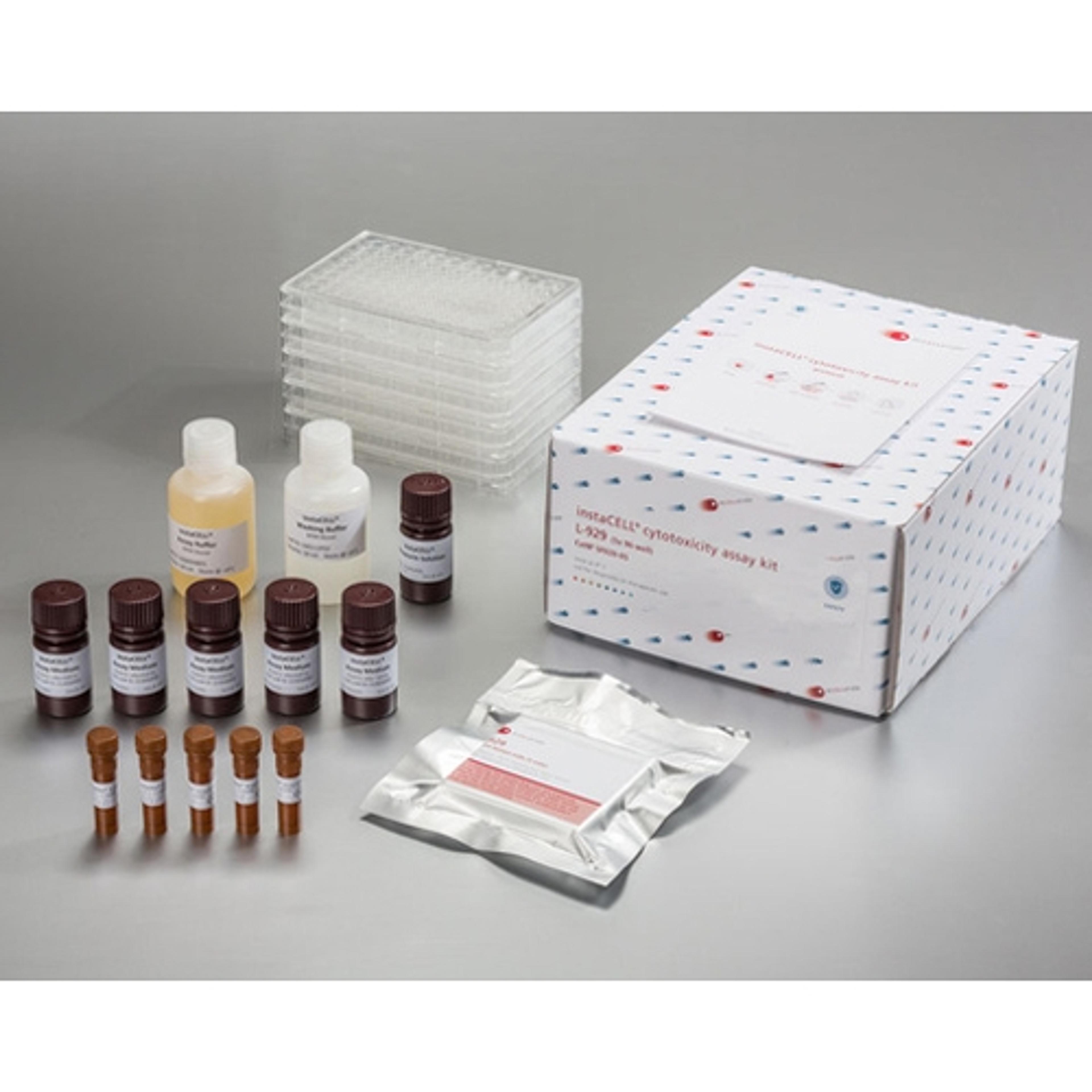
instaCELL Biocompatibility Assay Kit
Cell based kit including assay ready L-929 cells to measure biocompatibility of medical devices

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
The biocompatibility of medical devices needs to be assessed as laid down in the ISO 10993 guideline. This include the cytotoxicity testing of extracts prepared from the material on L-929 fibroblast cells.
The biocompatibility assay kit is built on assay ready L-929 cells which can be used instantly for the test without pre-cultivation. Briefly, the cells are thawed, seeded into the provided assay plates and challenged with the extracts in serial dilutions. Viability of the cells is quantified by the addition of XTT, a metabolic dye for a colorimetric read-out, which is included in the kit as well as appropriate references materials.
- Includes reliably performing assay ready cells
- No cell cultivation required
- Improved reproducibility by using cells like a standardized reagent