Interferon Antibody Bioassays Services
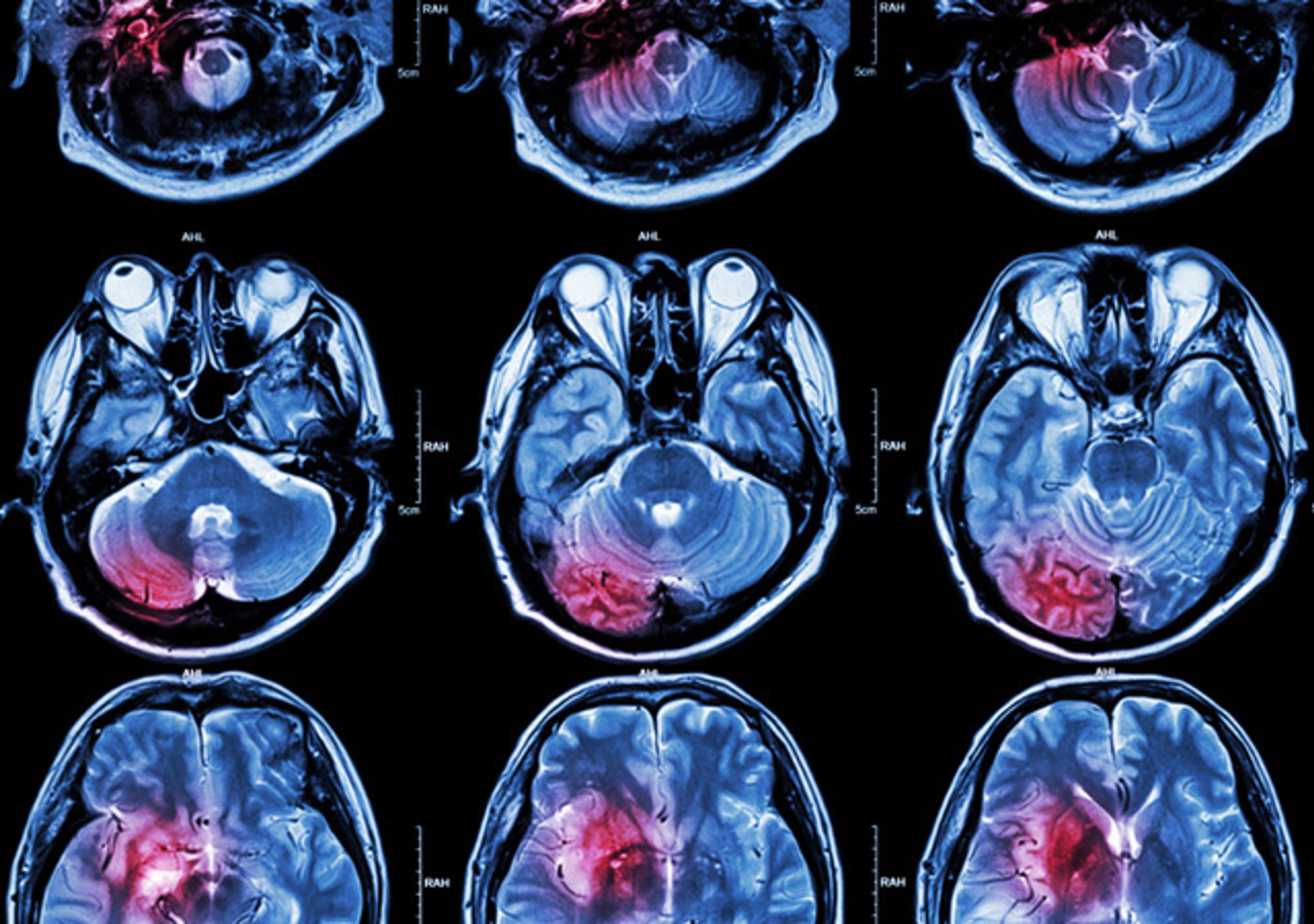
PBL's bioassays for interferons are cytopathic effect inhibition assays, also known as CPE and antiviral assays. The neutralization assay measures the antibody's ability to neutralize the CPE activity of the cytokine. Sample types include; tissue culture supernatents; serum and delivery formulations. In these assays, one unit is defined as the quantity of interferon required per milliliter to reduce the cytopathic effect of vi…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
PBL's bioassays for interferons are cytopathic effect inhibition assays, also known as CPE and antiviral assays. The neutralization assay measures the antibody's ability to neutralize the CPE activity of the cytokine. Sample types include; tissue culture supernatents; serum and delivery formulations. In these assays, one unit is defined as the quantity of interferon required per milliliter to reduce the cytopathic effect of viral infection by 50%. All standards used in our bioassays have been calibrated to the International Reference Standards such as those outlined by the National Institutes of Health [Pestka, S. (1986) Methods of Enzymology, 119, 14-23] and the World Health Organization. Results are normally provided 2-3 weeks after receipt of samples, although assay times may vary.
Neutralization assays are offered for Human, Mouse, Rat and Monkey inteferon alpha, beta and gamma. The assay system, specifically the cell and virus types, varies depending on the derived species of interferon to be analyzed.