Oncomine Dx Target Test for Non-Small Cell Lung Cancer
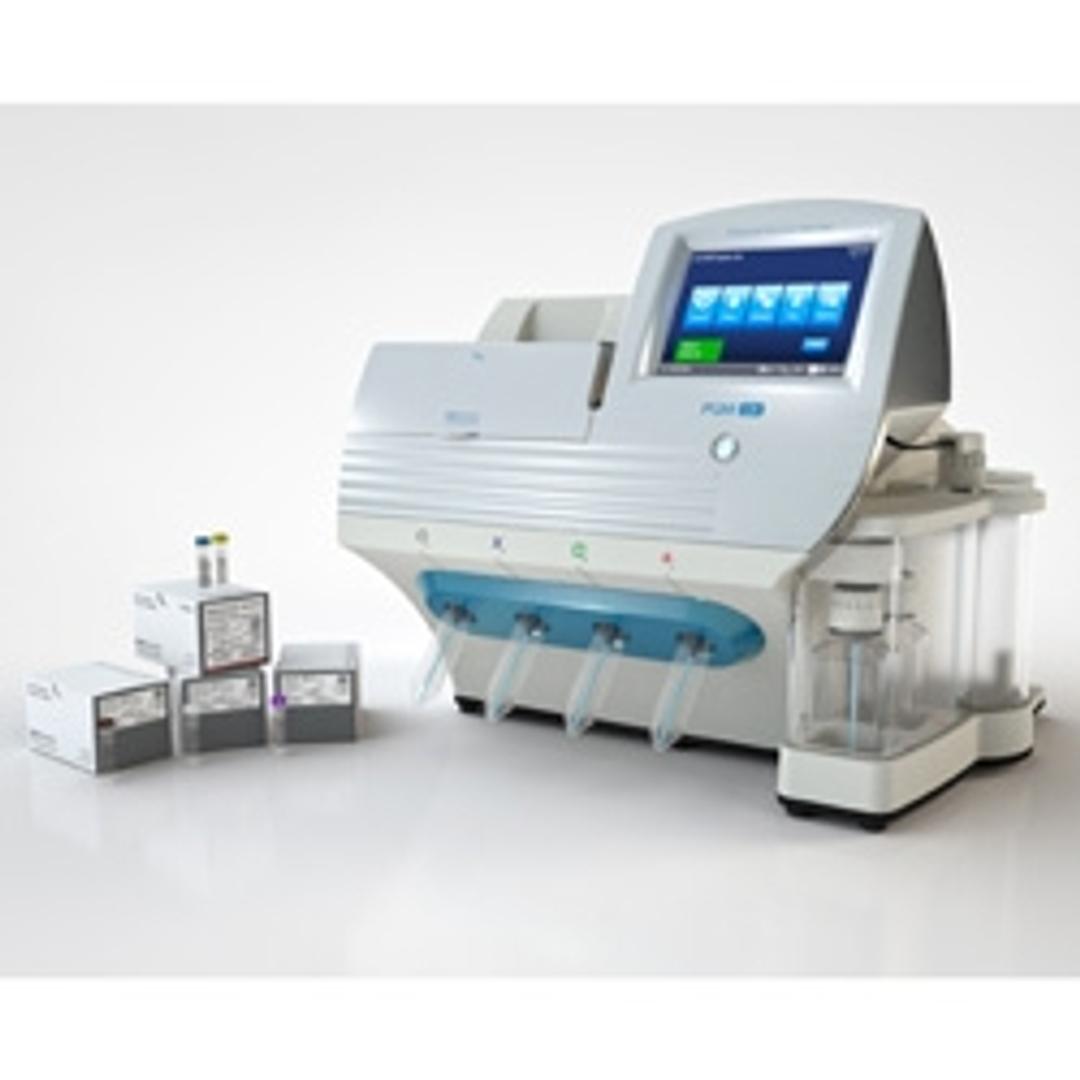
One test that can expedite NSCLC treatment selection decisions. The Oncomine Dx Target Test is the first targeted NGS-based in vitro diagnostic test for NSCLC, simultaneously delivering multiple biomarker results from one sample within four days and helping inform treatment decisions.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
It is the best way to use and to see in the patient
diagnostic testing for NSCLC
One test that can expedite NSCLC treatment selection decisions. The Oncomine Dx Target Test is the first targeted NGS-based in vitro diagnostic test for NSCLC, simultaneously delivering multiple biomarker results from one sample within four days and helping inform treatment decisions. It is the best way to use and to see in the patient
Review Date: 31 Jul 2018 | Thermo Fisher Scientific
Simple reaction setup, downstream data analysis and clinical relevance.
Clinal testing
Simple reaction setup, downstream data analysis and clinical relevance also easy to find and assign.
Review Date: 25 Jul 2018 | Thermo Fisher Scientific
In the era of personalized medicine, molecular profiling has become essential for the treatment of patients with metastatic non-small cell lung carcinoma (NSCLC). With an increasing number of genomic alterations becoming clinically relevant, sequential testing of individual mutations becomes a significant challenge for clinical laboratories. Next-generation sequencing (NGS), which can detect multiple alterations at once from a small amount of tissue, offers a solution.
One test that can expedite NSCLC treatment selection decisions
The Ion Torrent Oncomine Dx Target Test is the first targeted NGS-based in vitro diagnostic test for NSCLC, simultaneously delivering multiple biomarker results from one sample within four days and helping inform treatment decisions.
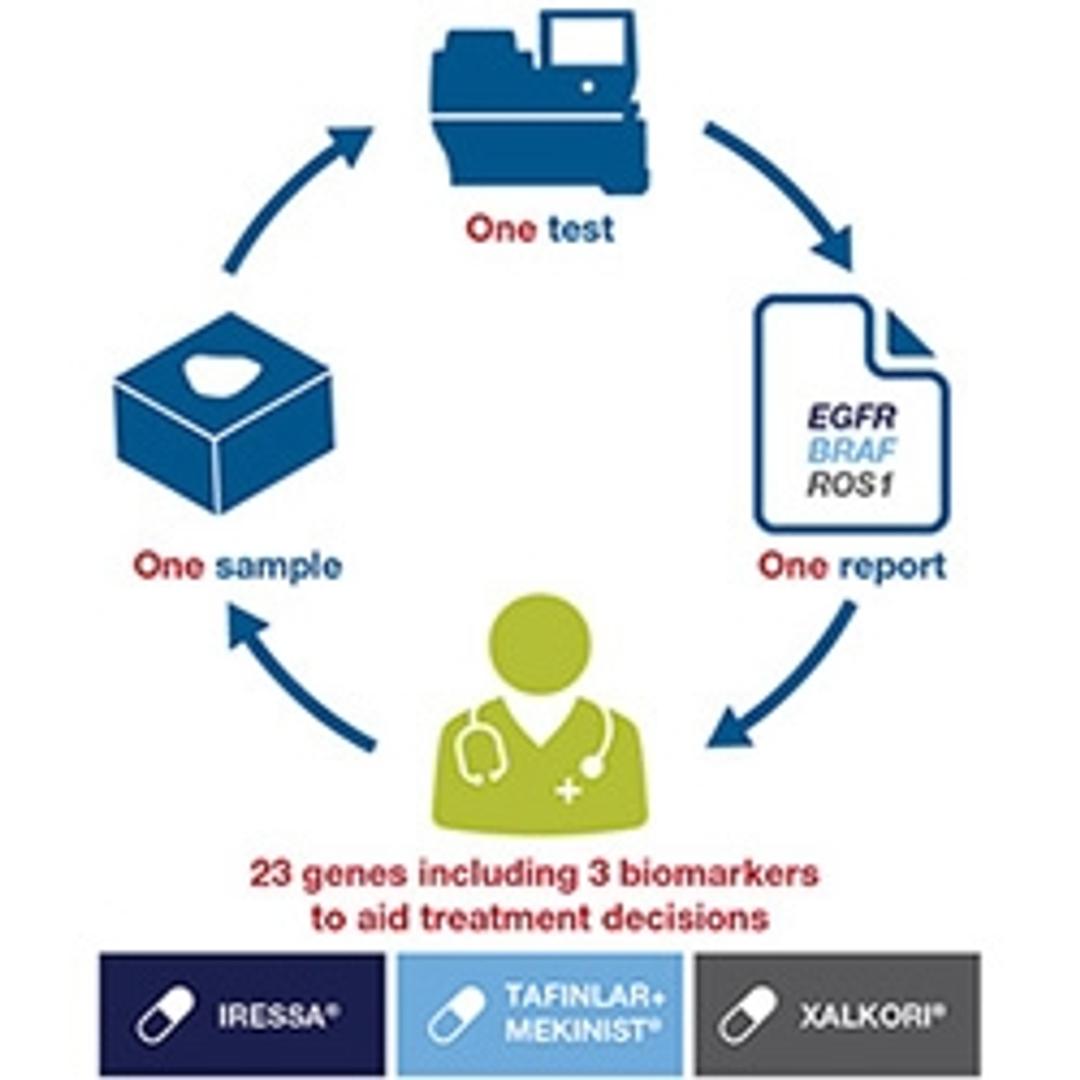
- Multiple therapies—one test indicated as a companion diagnostic device to aid in selecting NSCLC patients for treatment with targeted therapies, including IRESSA® (gefitinib) for EGFR L858R and exon 19 deletion, TAFINLAR + MEKINIST® (dabrafenib in combination with trametinib) for BRAF V600E, or XALKORI® (crizotinib) for ROS1 fusion
- Multiple biomarkers—one test for detection of 368variants in 23 cancer-associated genes that are clinically associated with NSCLC
- One sample—one sample used to deliver multiple biomarker results, minimizing the risk of depleting tissues and requiring additional biopsies
- Fast results— From sample extraction to clinical test report, the total workflow turn-around time is 4 days
- Automated clinical report—The Oncomine Dx Target Test results are presented in a single, two-part Clinical Test Report that incorporates NSCLC biomarker results, associated therapy indication(s), and other biomarker results
- Established performance—Concordance with comparator methods based on FISH or PCR was established for EGFR, BRAF and ROS1
Oncomine Dx Target Test content
Currently, five NSCLC-associated biomarkers are targeted by first line treatment. Additional biomarkers have been recommended by scientific guidelines for adding potential value in the patient stratification process. The Oncomine Dx Target Test is the only available diagnostic test that delivers multiple biomarker identification at once. The test includes three biomarkers, validated for selection of relevant targeted therapies (EGFR, ROS1, or BRAF), and twenty additional genes, relevant for NSCLC pathogenesis analytically validated for variant detection from NSCLC tissue.
The power of next generation sequencing
Next-generation sequencing (NGS) can sequence hundreds to thousands of genes and detect multiple biomarkers at the same time. The sequencing takes place in a chip that contains millions of wells (flow cells) with separate sequencing reactions taking place in each well, allowing many genes to be sequenced at once and multiple variations to be detected simultaneously, unlike traditional companion diagnostic technologies such as FISH, IHC, or PCR, which only analyze one target gene at the time.
Oncomine Dx Target Test report
The Oncomine Dx Target Test Clinical Test Report is automatically generated as a PDF and incorporates relevant patient, sample, and test information required to help ensure high-performance standards, regulatory compliance, and quality control. The test results are presented in two-parts: companion diagnostic marker results with associated therapy indications and cancer driver analytical-only biomarker results in a separate section. The report is customizable and LIMS system-compatible.