PDX Mouse Clinical Trials (MCTs)
Accelerate oncology drug development with Crown Bioscience’s PDX Mouse Clinical Trials. Leverage the world’s largest clinically relevant PDX collection to model patient tumor heterogeneity, predict clinical outcomes, discover biomarkers, and optimize drug strategies. Improve clinical translation and reduce attrition with highly predictive, patient-relevant insights.
Bridging the Gap Between Preclinical Research and Clinical Success
In the complex landscape of oncology drug development, the high attrition rate of therapies—particularly in Phase II trials—underscores the need for more predictive preclinical models. Crown Bioscience addresses this challenge with its Patient-Derived Xenograft (PDX) Mouse Clinical Trials (MCTs), offering a transformative approach to preclinical oncology research.
What Are PDX Mouse Clinical Trials?
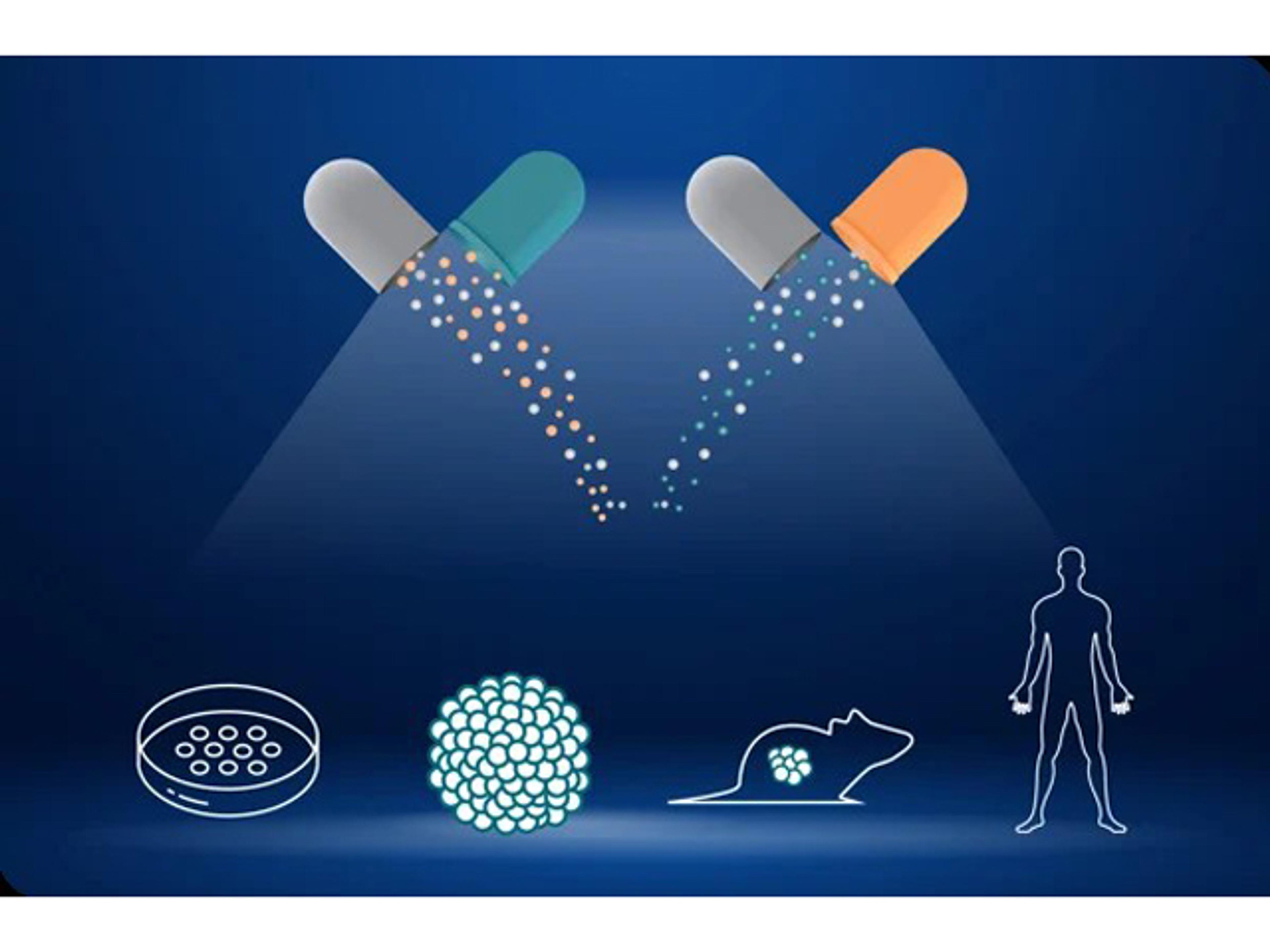
PDX Mouse Clinical Trials are preclinical studies that emulate human clinical trials by implanting patient-derived tumor tissues into immunodeficient mice. This methodology preserves the genetic and phenotypic characteristics of the original tumors, providing a more accurate representation of human cancer biology. By testing drug candidates across diverse PDX models, researchers can gain insights into efficacy, resistance mechanisms, and potential biomarkers in a setting that closely mirrors patient heterogeneity.
Key Advantages of PDX Mouse Clinical Trials:
Enhanced Clinical Translation: By maintaining the key features of patient tumors, PDX models offer more accurate predictions of clinical outcomes, thereby improving the likelihood of success in subsequent human trials.
Predictive Biomarker Discovery: These models facilitate the identification and validation of biomarkers for patient stratification, enabling more personalized treatment strategies.
Exploration of New Indications: PDX models allow for the evaluation of therapies across various cancer types, uncovering potential new therapeutic applications.
Deeper Drug Response Insights: Gain a comprehensive understanding of drug efficacy, mechanisms of action, and resistance mechanisms, which are invaluable for optimizing drug combinations and reducing attrition in clinical development.
Comprehensive Support from Trial Design to Data Analysis
Crown Bioscience provides end-to-end services for PDX Mouse Clinical Trials, including:
- Expert Trial Design: Customized study designs to maximize predictive power and ensure clinical relevance.
- Integrated Data Reports: Comprehensive reports combining biomarker discovery, pharmacokinetic/pharmacodynamic (PK/PD) analysis, and bioinformatics.
- Seamless Execution: Dedicated teams ensure consistency, quality, and efficiency at every stage of the trial.
Applications in Oncology Research:
- PDX Mouse Clinical Trials support diverse oncology research areas, including:
- Biomarker Discovery & Validation: Identifying biomarkers for personalized treatment strategies.
- Clinical Stratification: Generating precise data to support targeted therapies and improve patient selection.
- Exploring New Indications: Investigating therapies across various cancer types to discover new therapeutic uses.
- Targeted Research: Focusing on specific genetic mutations or cancer drivers to refine therapeutic approaches.
- Drug Combination Strategies: Evaluating drug combinations to overcome resistance and optimize treatment responses.