Preclinical Biomarker Services
Crown Bioscience’s preclinical biomarker services help identify, validate, and analyze biomarkers across diverse in vitro and in vivo cancer models. Using multi-omics platforms and expert bioinformatics, these services support drug mechanism studies, patient stratification, and companion diagnostic development to guide oncology drug discovery.
Comprehensive Preclinical Biomarker Discovery for Oncology Drug Development
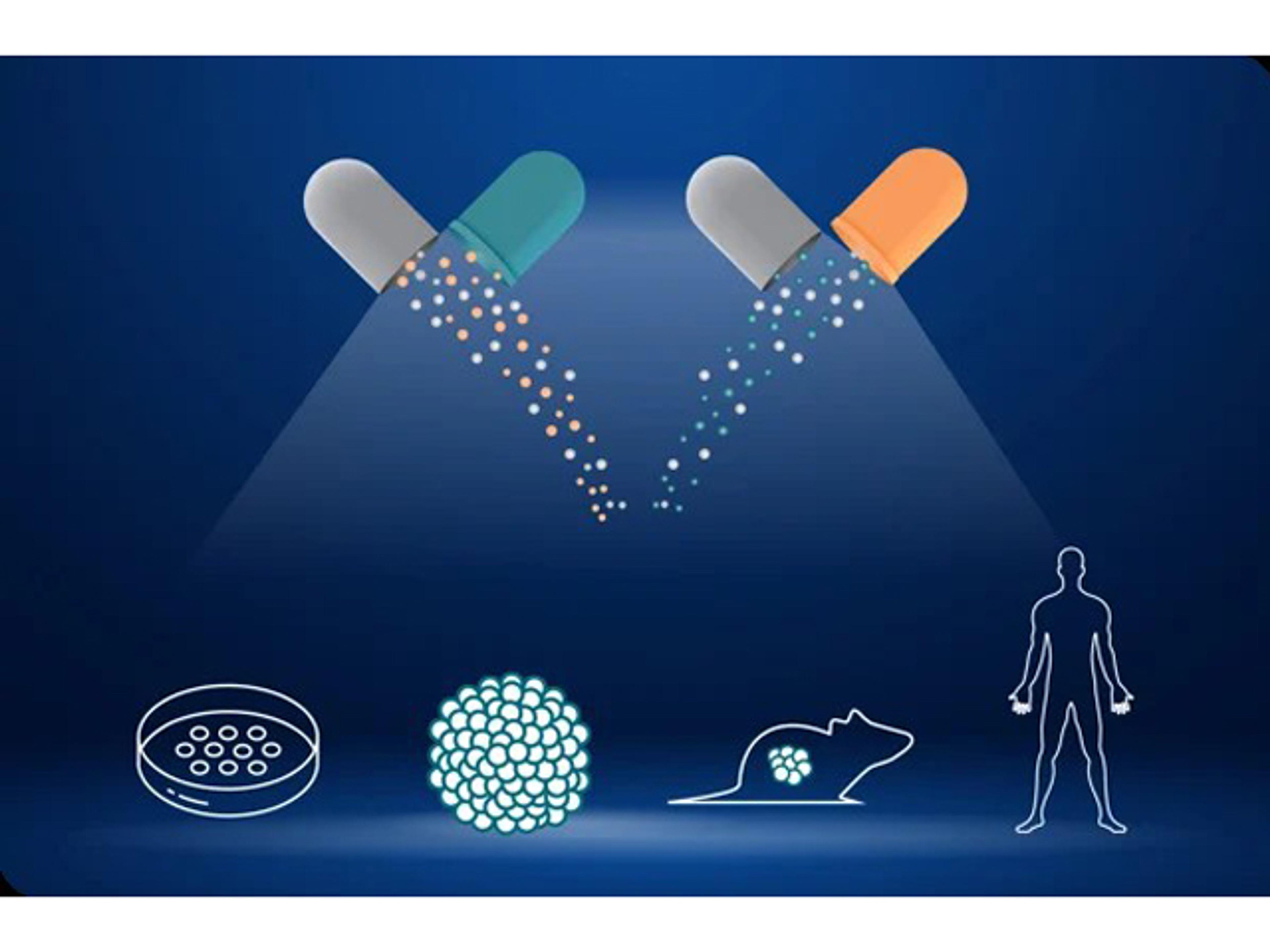
In the pursuit of personalized cancer therapies, the identification and validation of predictive biomarkers are paramount. Crown Bioscience offers a robust preclinical biomarker discovery platform, integrating in silico, in vitro, ex vivo, and in vivo methodologies to support oncology drug development.
Key Components of the Platform:
- Extensive Cancer Model Repository: Utilize over 1,000 cancer cell lines, 600 organoids, and 2,600 patient-derived xenograft (PDX) models to capture tumor heterogeneity and enhance translational relevance.
- Flexible Screening Options: Tailor screening approaches to specific research phases, from high-throughput cell line assays to advanced PDX mouse clinical trials, enabling efficient biomarker identification and validation.
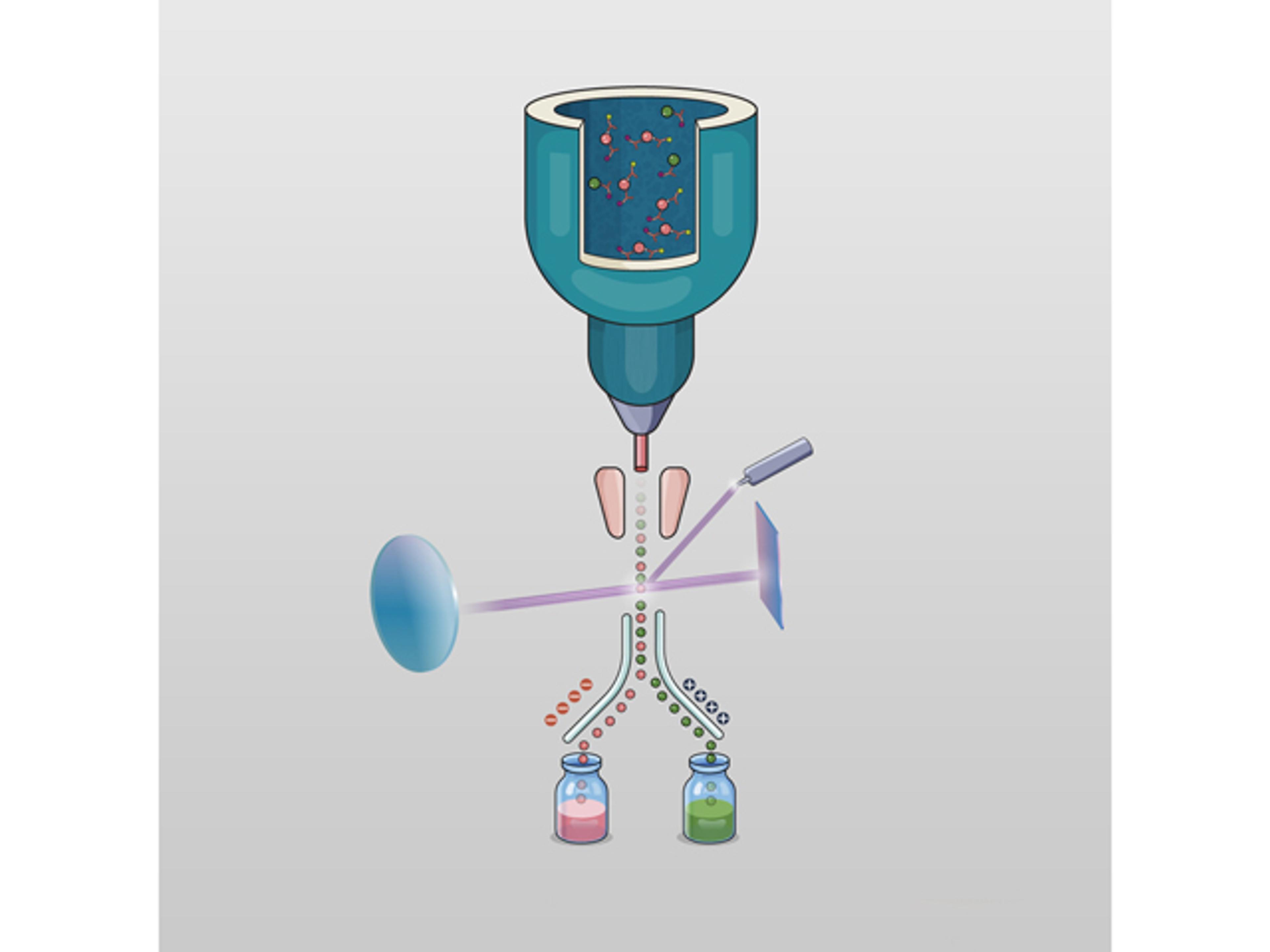
- Multi-Omics Integration: Combine genomic, transcriptomic, proteomic, and metabolomic data to uncover comprehensive biomarker profiles and elucidate mechanisms of action.
- In-House Bioinformatics Expertise: Leverage specialized bioinformatics support for study design, data analysis, and interpretation, ensuring actionable insights and informed decision-making.
Applications in Drug Development:
- Predictive Biomarker Identification: Discover biomarkers that can stratify patient populations and predict therapeutic responses, enhancing clinical trial design and success rates.
- Mechanism of Action Studies: Investigate the biological pathways influenced by therapeutic agents to understand their effects and potential resistance mechanisms.
- Companion Diagnostic Development: Develop and validate diagnostic tools alongside therapeutics to identify suitable patient cohorts.