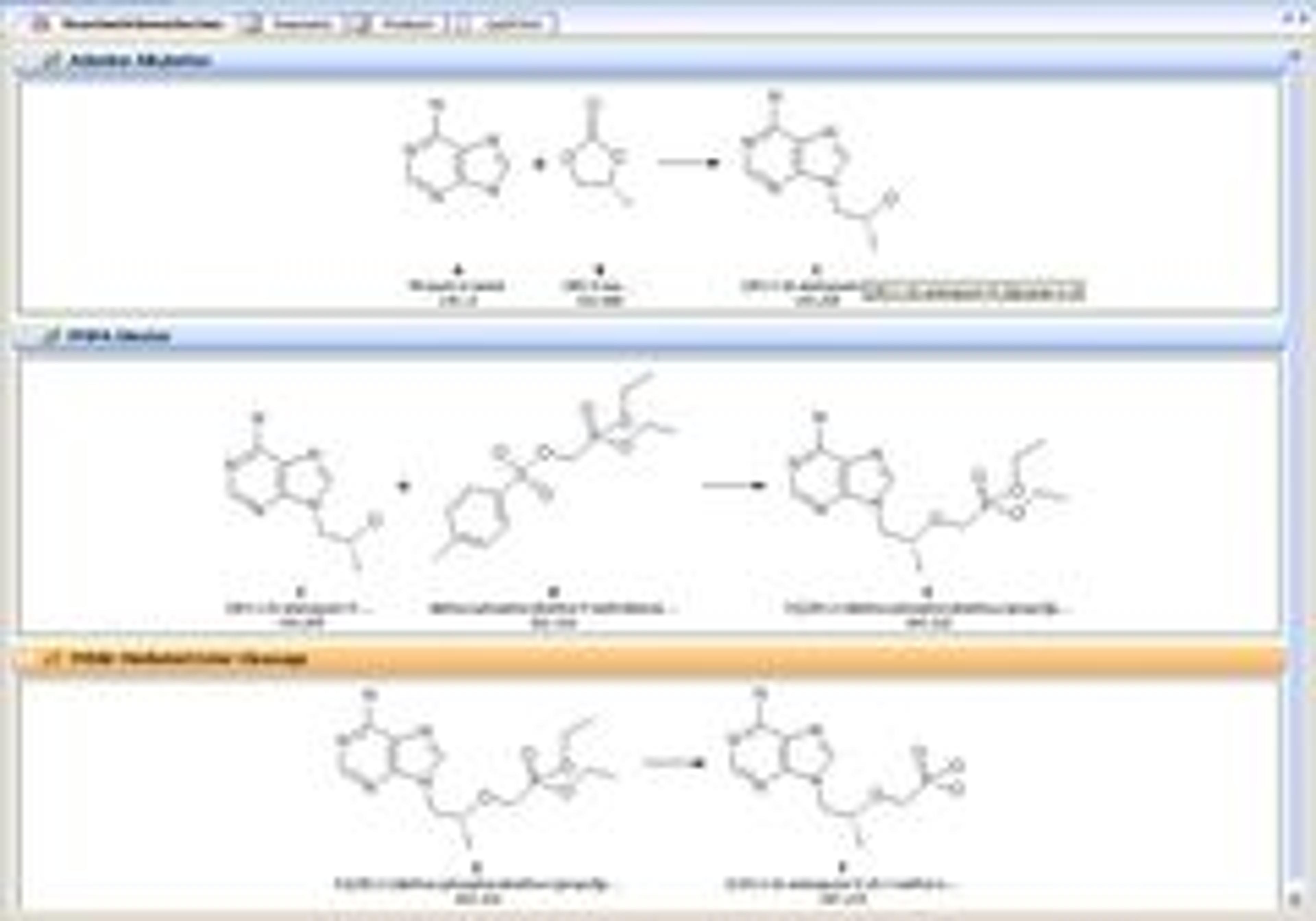
Process E-Lab Notebook
Symyx Software Process Notebook is an Electronic Laboratory Notebook (ELN) designed specifically to solve the challenges faced by process chemists, improving information management, saving time, and improving experiment quality. Prepare: Efficiently Plan Experiments Searchable global database of corporate knowledge enables scientists to make informed decisions and design better experiments. Document editors specificall…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Symyx Software Process Notebook is an Electronic Laboratory Notebook (ELN) designed specifically to solve the challenges faced by process chemists, improving information management, saving time, and improving experiment quality.
Prepare: Efficiently Plan Experiments
- Searchable global database of corporate knowledge enables scientists to make informed decisions and design better experiments.
- Document editors specifically designed for process chemists utilize automated stoichiometric and scaling calculations to simplify experimental setup.
- Ability to plan and execute parallel experiments enables faster and better process optimization.
Document: Record Experiments and Create GMP Documentation
- Customizable QuickText and a built-in database of chemicals and associated properties enable rapid, convenient procedure entry to improve information quality.
- Automatic generation of batch records and process flow diagrams saves time and streamlines the generation of GMP documentation.
Analyze: Rapidly Evaluate Chemical Process Information
- Standardized and accessible documentation of all chemical process information streamlines technology transfer across the clinical supply chain and to outsourcing partners.
- Multi-step process planner provides detailed campaign planning, including the analysis of materials, resources, waste disposal, and overall costs.
- A single Notebook platform spanning discovery and development, facilitates rapid data aggregation for reports, including FDA submissions.