PureFlex™ process container film
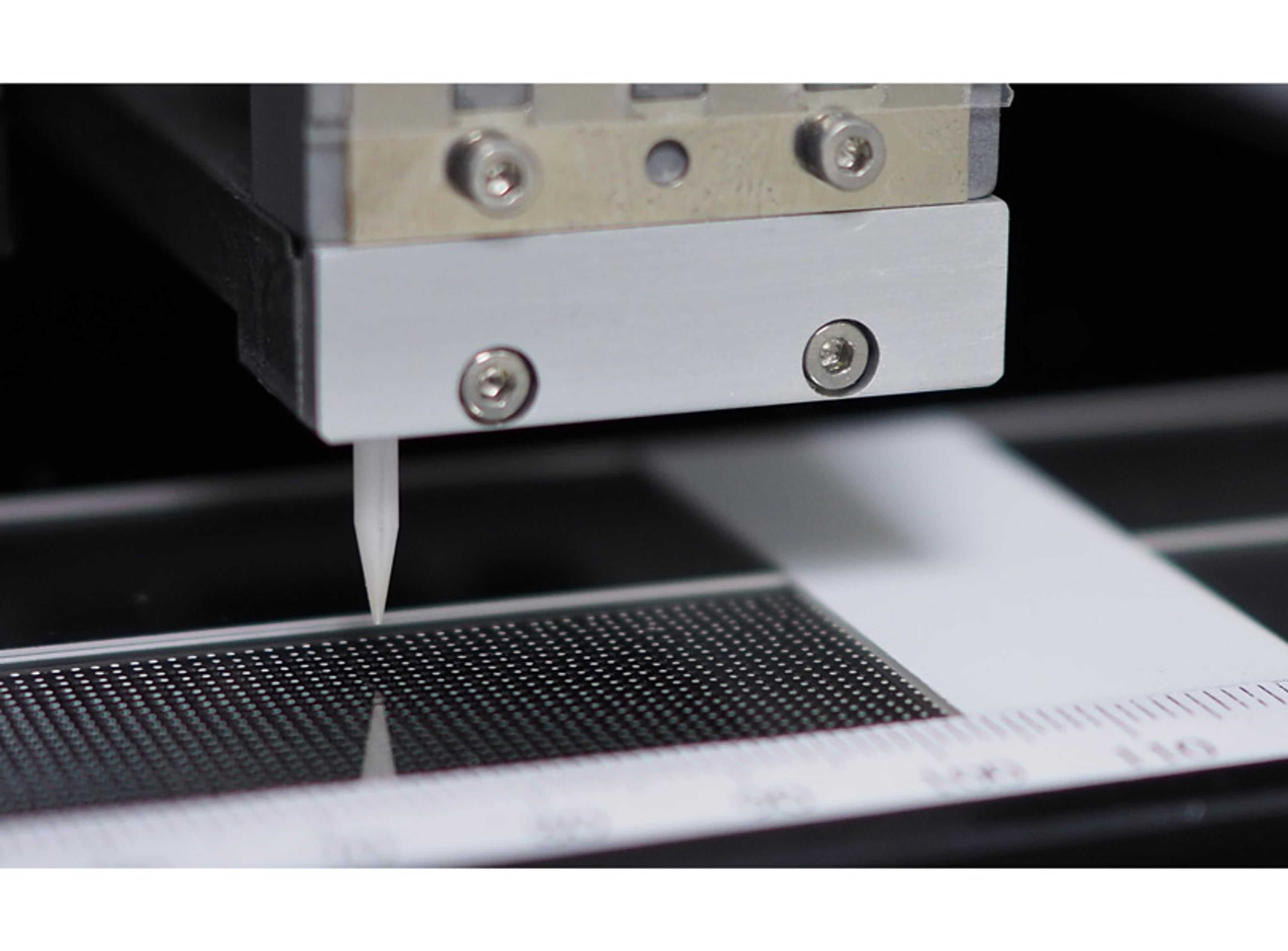
Designed to provide strength, flexibility (with maximum resistance to flex-crack), excellent gas barrier performance and inert contact. PureFlex™ Single-use process container films are flexible, robust and chemical resistant materials used in the construction of our single-use process containers, including Mobius® assemblies and NovaSeptum® sterile sampling products.PureFlex film is a high purity, medical grade, coextruded fi…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Designed to provide strength, flexibility (with maximum resistance to flex-crack), excellent gas barrier performance and inert contact.
PureFlex™ Single-use process container films are flexible, robust and chemical resistant materials used in the construction of our single-use process containers, including Mobius® assemblies and NovaSeptum® sterile sampling products.
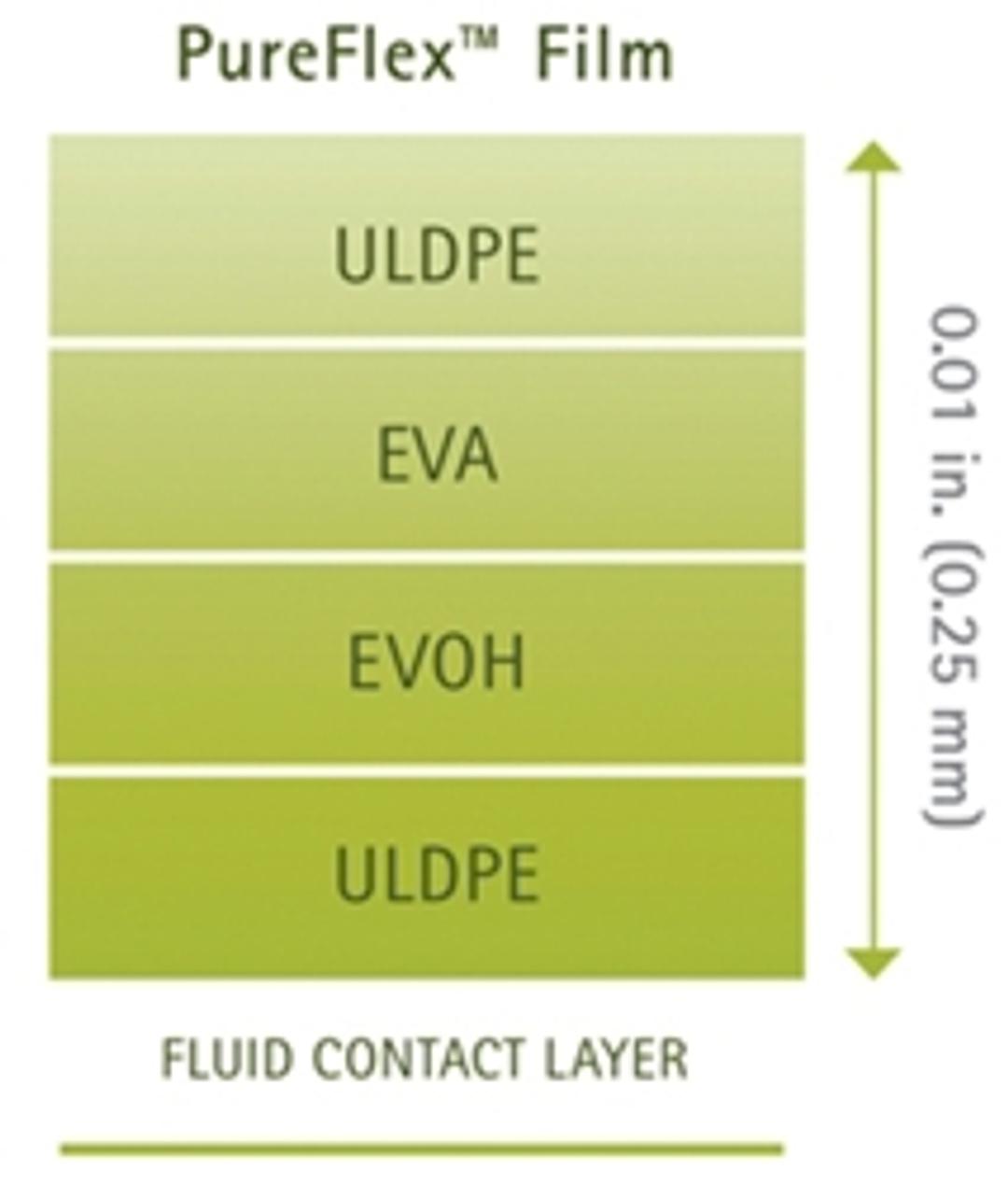
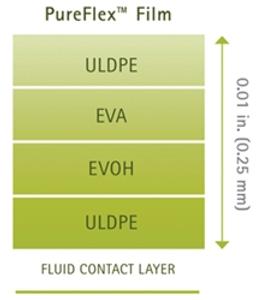
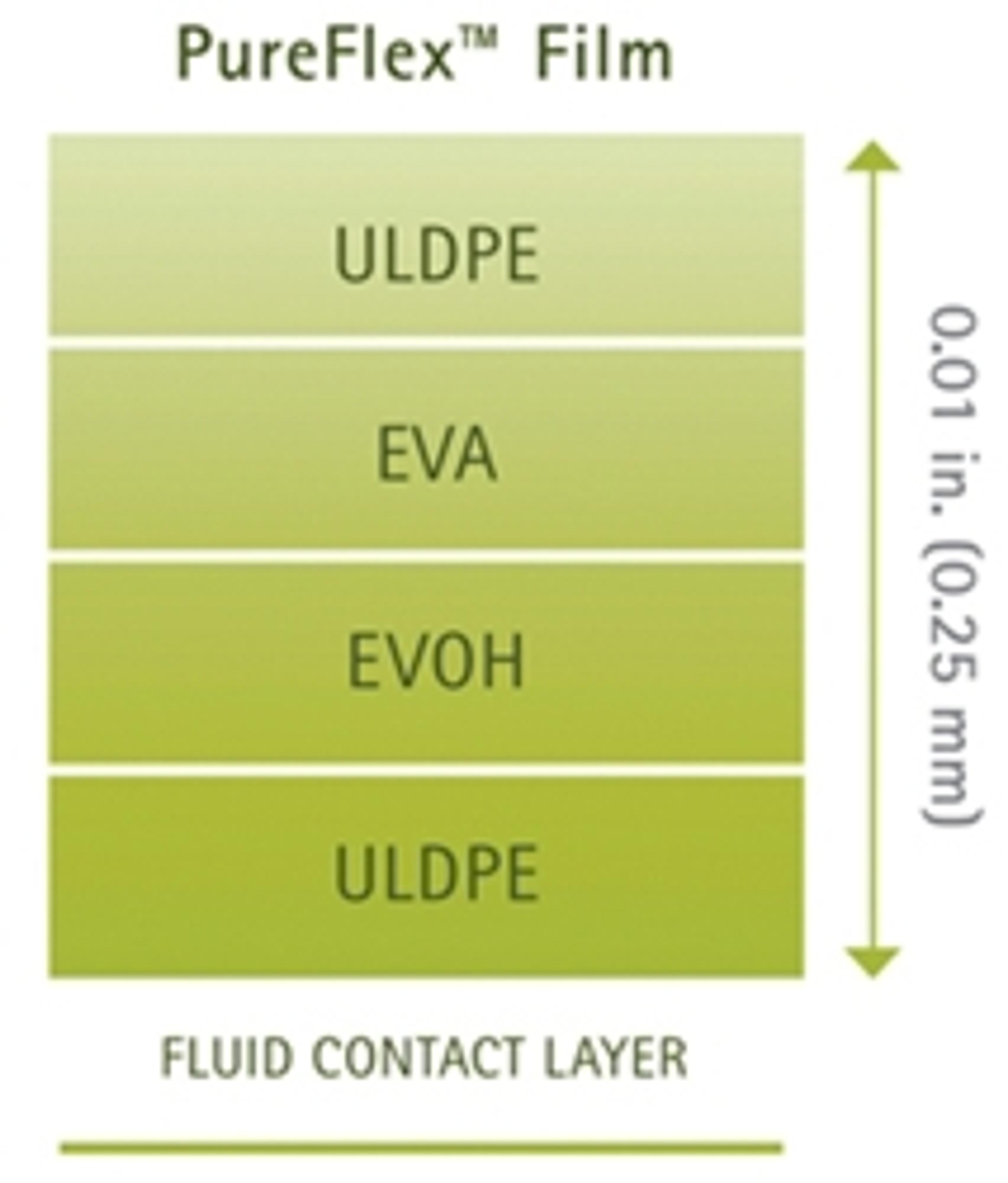
PureFlex film is a high purity, medical grade, coextruded film designed to provide strength, flexibility (with maximum resistance to flex-crack), excellent gas barrier performance and inert contact. The fluid contact material is made of ultra low density polyethylene (ULDPE). The gas barrier is made of polyethylene vinyl alcoholcopolymers (EVOH). The outer layers are made of ethylene vinyl acetate (EVA) and ULDPE. PureFlex film contact layers comply with the Food and Drug Administration (FDA) regulation 21 CFR 177.1520.
For particularly demanding applications often encountered in large volume operations (>500L), additional robustness may be required to assure single-use process container integrity. PureFlex™ Plus film is constructed with a tough, linear low density polyethlene (LLDPE) outer layer. This rugged outer layer increases the film's resistance to leak formation through abrasion, puncture, stretching, and tearing. The inner layers, including the product contact layer, are identical to PureFlex™ film, maintaining the same extractables profile and gas barrier properties.