Ready 4 Action Product Planning Service
Ready 4 Action, proprietary feasibility assessment, sets the cornerstone of a cost-effective product development plan. Ready 4 Action is the four-step process used to evaluate the viability of proposed drug products. This important tool enables clients to make the go/no-go decision regarding a given drug's market potential and clinical development feasibility.Armed with the neccessary intelligence, uncover with Ready 4 Action…
Ready 4 Action, proprietary feasibility assessment, sets the cornerstone of a cost-effective product development plan.
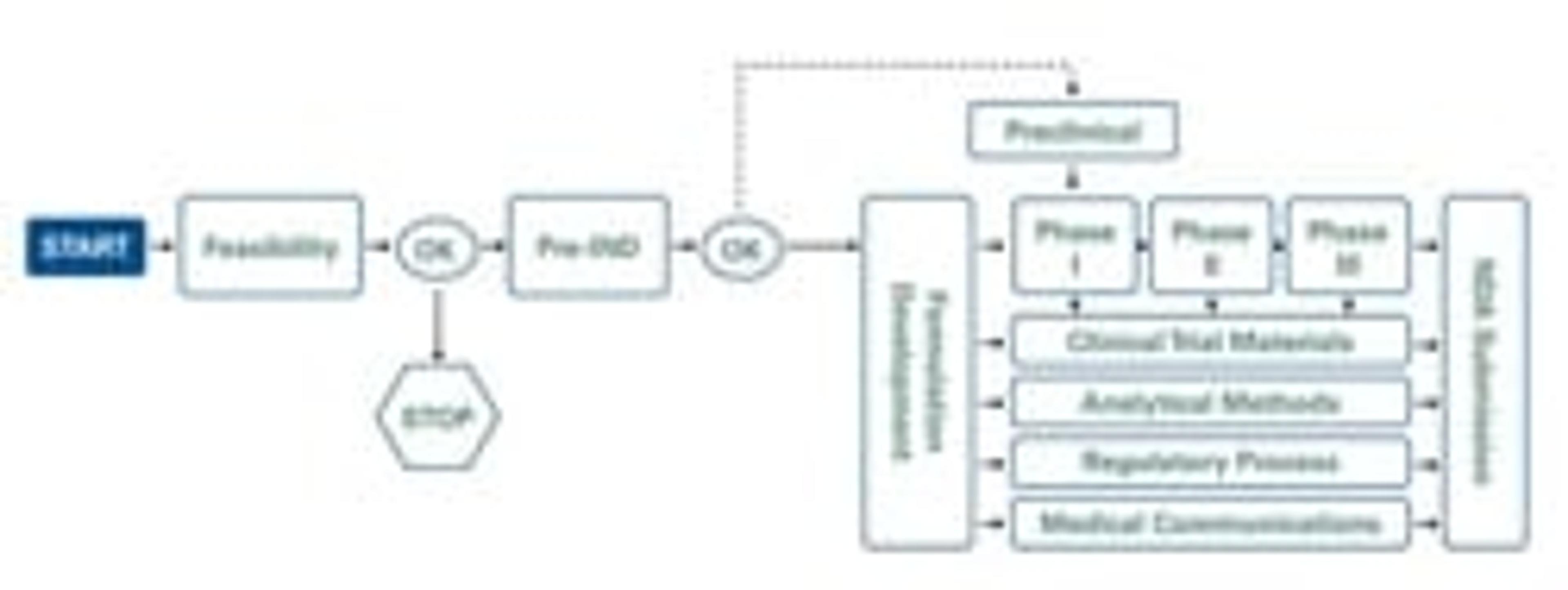
Ready 4 Action is the four-step process used to evaluate the viability of proposed drug products. This important tool enables clients to make the go/no-go decision regarding a given drug's market potential and clinical development feasibility.
Armed with the neccessary intelligence, uncover with Ready 4 Action — including details of key factors influencing the pharmaceutical marketplace, the regulatory status and the clinical strategy involved in development of a specific drug — clients can make well-informed drug development decisions and avoid unnecessary and potentially destructive financial risks.
Camargo's Ready 4 Action assessment includes detailed data pertaining to your specific drug, along with a proposed clinical development plan — an invaluable feature of the assessment — and a schematic of the proposed plan. Incorporating major decision points and cost estimates, your plan includes clinical pharmacology, preclinical studies, pharmacokinetics and regulatory recommendations — that’s why it is a critical first step in the race to drug approval.
Ready 4 Action Product Planning Service Assessment:
Scientific Viability - Does the science make sense? For instance, is the formulation or chemistry practically and pragmatically achievable? Is it scalable? Are API ingredients available and affordable?
Medical Viability - Does the product have a clear niche in the medical specialty? Is it effective for solving a unique problem or solving a problem in a unique way? Does it present an acceptable risk/benefit? Is it appealing to the proposed patient population?
Regulatory Viability - What clinical trials or other data will be required to gain approval? Can development be expedited? What distinguishing information can be presented on the labeling for eventual promotional activity?
Commercial Viability - Is there a viable market for the product? What is the potential for future competition or substitution? What is needed to ensure reimbursement? What is the optimal pricing?