Products & ReviewClinical Diagnostics
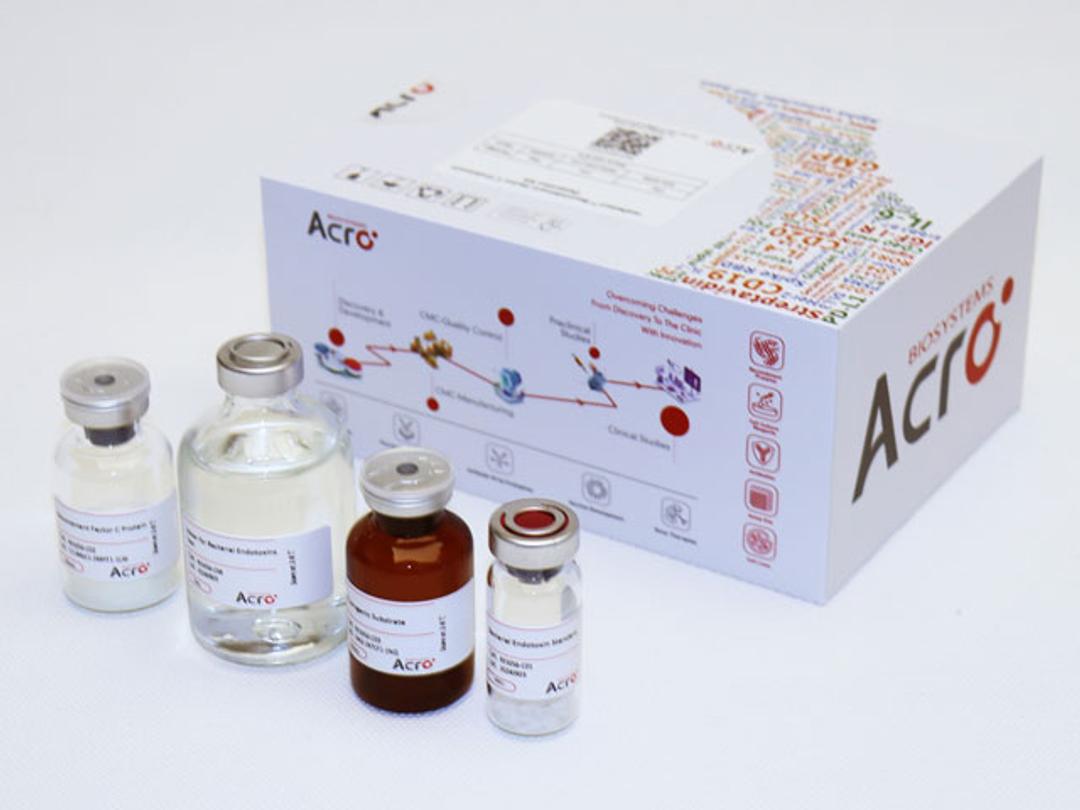
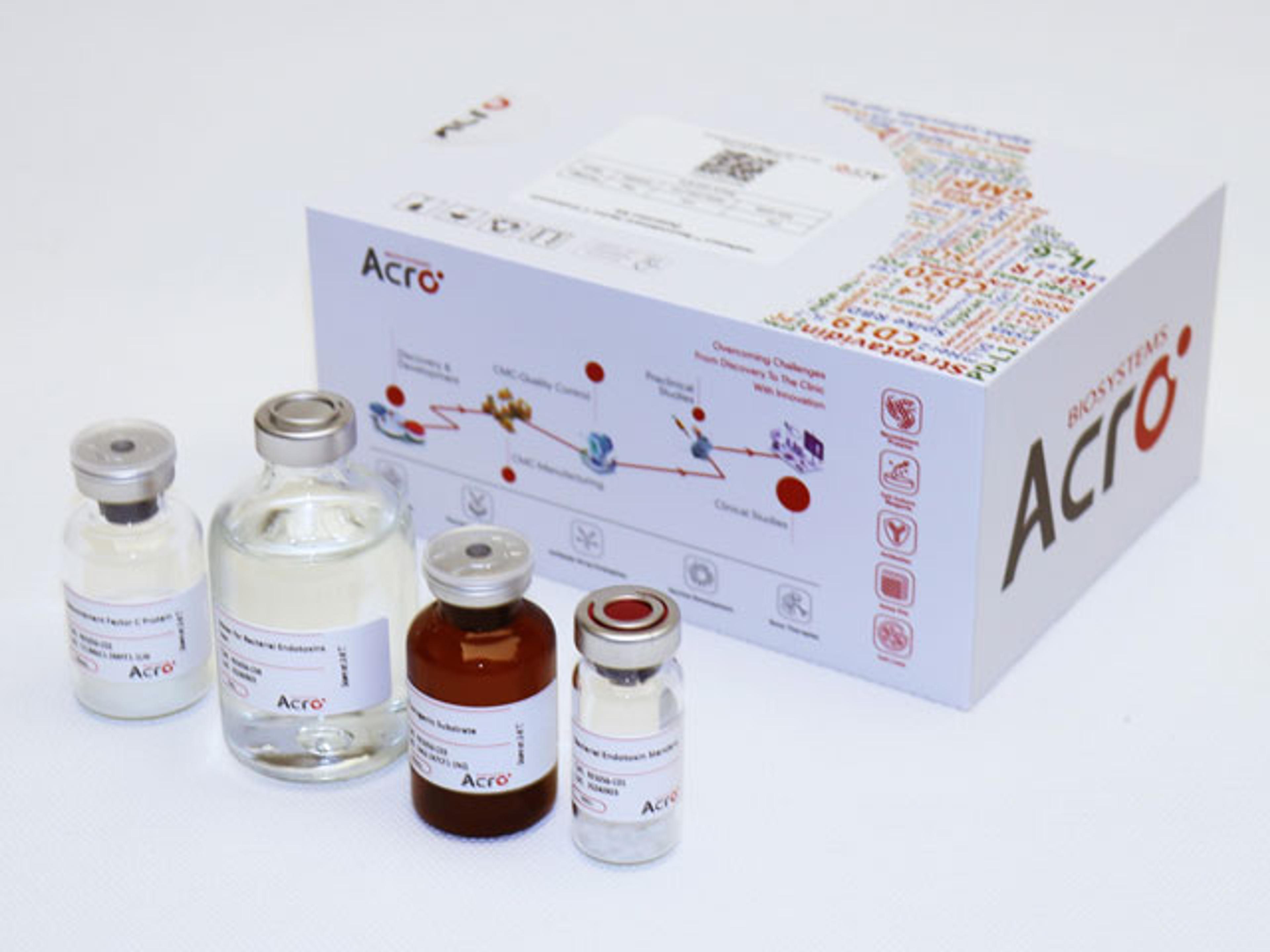
Recombinant Factor C Endotoxin Detection Kit
ACROBiosystems' Recombinant Factor C Endotoxin Testing Kit (Cat. NO. RES-A056) offers a sustainable alternative to traditional LAL testing by eliminating reliance on horseshoe crab blood. The rFC assay ensures regulatory compliance, supports animal welfare, and aligns sustainability goals, making it a superior choice for environmentally conscious endotoxin testing. Developed through proprietary platform technology, this endpoi…
- Comparable to LAL method - Endpoint fluorescent assay, comparable to other chromogenic quantitative LAL methods
- High specificity - Unlike LAL Assay, as Factor G is absent from the rFC test kit, false-positive results due to β-glucan activation are not expected to occur.
- Accuracy - Traceability of endotoxin standards in the kit against USP Standard (Catalog No: 1235503).
- Fast time to results - 1 hours.
- High sensitivity - Sensitivity range from 0.005-5 EU/mL.
- Extensive validation – Verification on multiplex biological products, kinds of microplate readers and various buffer systems, comprehensive validation of specificity, sensitivity, precision, accuracy, applicability, and other aspects according to the parameters listed in the EUROPEAN PHARMACOPOEIA 11.0 and USP chapter<<1225>>.
- Sustainable resource - Get rid of dependence on animal derived reagents, reduce dependence on horseshoe crab resources and fishing pressure and realize long-term supply.
- Good inter batch consistency - Batch consistency of products is guaranteed due to the use of genetic recombination technology for production.