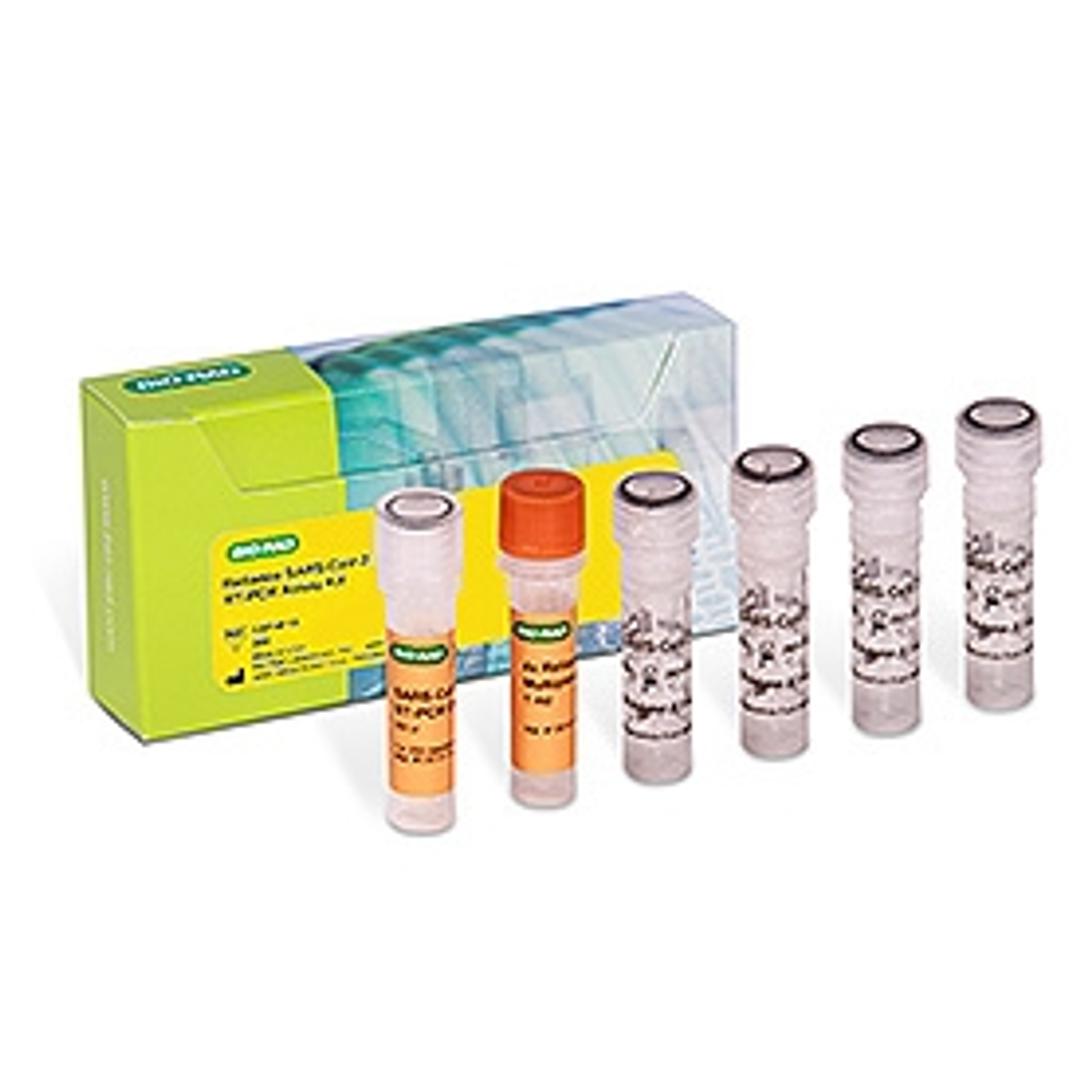
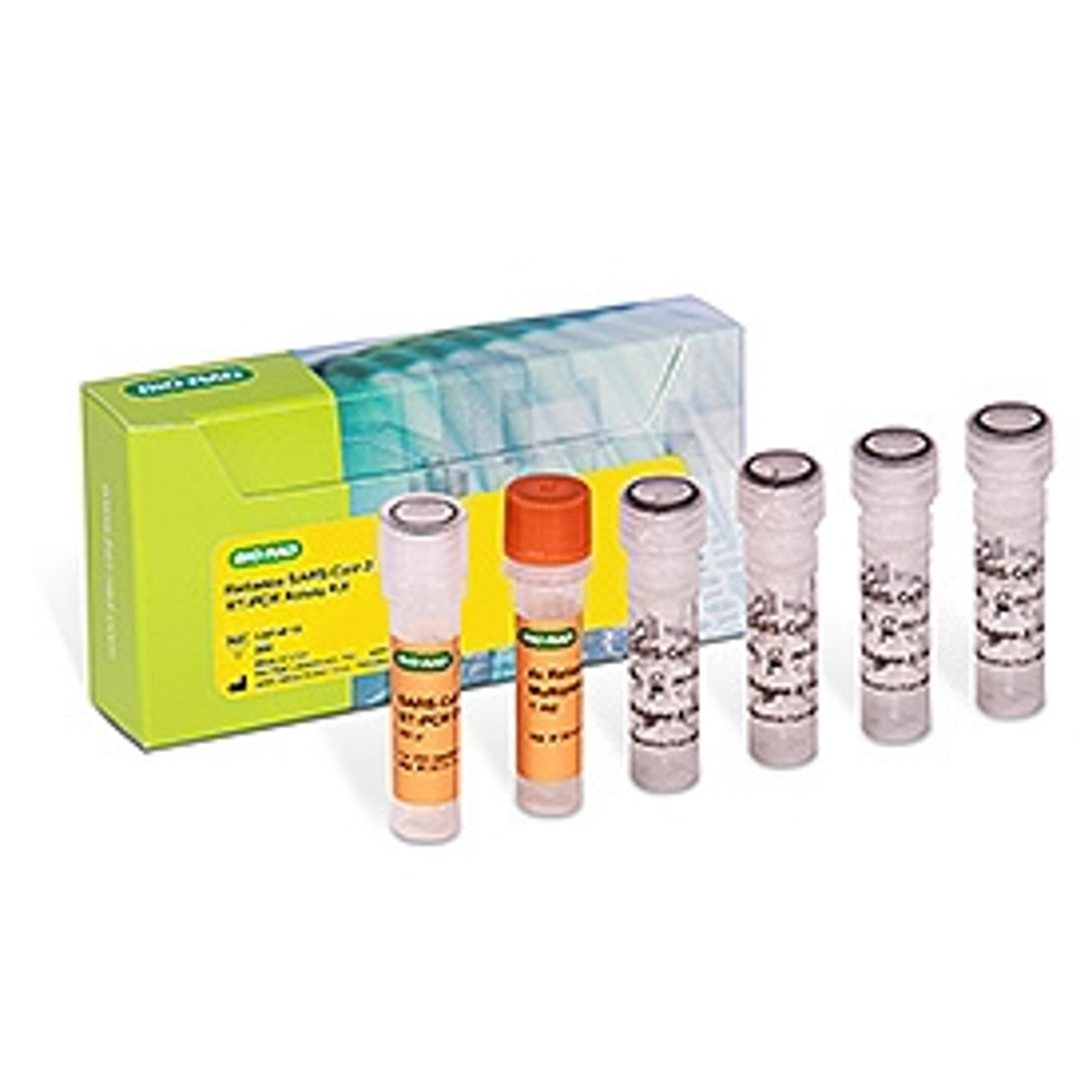
Reliance SARS-CoV-2 RT-PCR Assay Kit, 200 x 20 µl rxns #12014115
The Reliance SARS-CoV-2 RT-PCR Test provides real-time PCR detection of multiple RNA targets from the SARS-CoV-2 virus with great sensitivity and speed.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
The Reliance SARS-CoV-2 RT-PCR Test provides real-time PCR detection of multiple RNA targets from the SARS-CoV-2 virus with great sensitivity and speed. It is designed to detect nucleic acid from SARS-CoV-2 in nasopharyngeal swabs specimen from individuals suspected of COVID-19 by their healthcare provider.
Two primer/probe sets are the sequences reported by the Center for Disease Control and Prevention (CDC) for detection of SARS-CoV-2, selected from regions of the virus nucleocapsid (N1 and N2) genes. An additional primer/probe set to detect the human RNase P (RP) gene is also included in the panel as an internal control. RNA that is isolated and purified from control samples and clinical specimens is added to the master mix, which is composed of reverse transcriptase whereby RNA is converted into cDNA and then amplified, using the Bio-Rad Reliance One-Step Multiplex RT-qPCR Supermix.
The kit contains all materials necessary for the efficient detection of SARS-CoV-2, including independently developed Exact Diagnostics positive and negative molecular controls.
Features and Benefits
- Highly sensitive one-step RT-PCR workflow
- Specifically designed panel with three sets of primers/probes in a single multiplex assay
- Convenient kit package with full controls included
- Compatible with most popular PCR instrument platforms (CFX and AB7500 Systems)
Learn more about features, protocols, data interpretation, and fact sheets »
Kit Components
The kit contains all the required reagents for 200 reactions. The kit should be stored at –20 °C.
- SARS-CoV-2 RT-PCR Oligos
- Reliance One-Step Multiplex RT-qPCR Supermix
- Exact Diagnostics SARS-CoV-2 Standard
- Exact Diagnostics SARS-CoV-2 Negative
Related Products
- CFX Real-Time PCR Detection Systems
- CFX96 Dx Real-Time PCR Detection Systems for In-Vitro Diagnostics
- 96-Well and 384-Well PCR Plates
FDA Emergency Use Authorization:
This product has not been FDA cleared or approved, but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories; This product has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens; and, The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.