Sapio Clinical Diagnostics LIMS
Sapio LIMS for clinical diagnostics enables your lab to quickly design, deploy, and automate end-to-end workflows to meet your lab’s exact needs. Including a physician portal; automated accessioning and order processing; multithreaded, out-of-the-box, no/low-code configurable workflows; simple and sophisticated automation rules; and streamlined report generation.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Sapio LIMS for clinical diagnostics enables your lab to quickly design, deploy, and automate end-to-end operations designed precisely for your needs. Automate the entire clinical process from physician order creation and sample receipt/processing to final report creation, sign-off, and delivery. Best of all, Sapio can be tailored to your specific operational workflows and locations and can be configured to present essential data required by every role in your organization, reducing clicks and scrolling. Key features of the platform include:
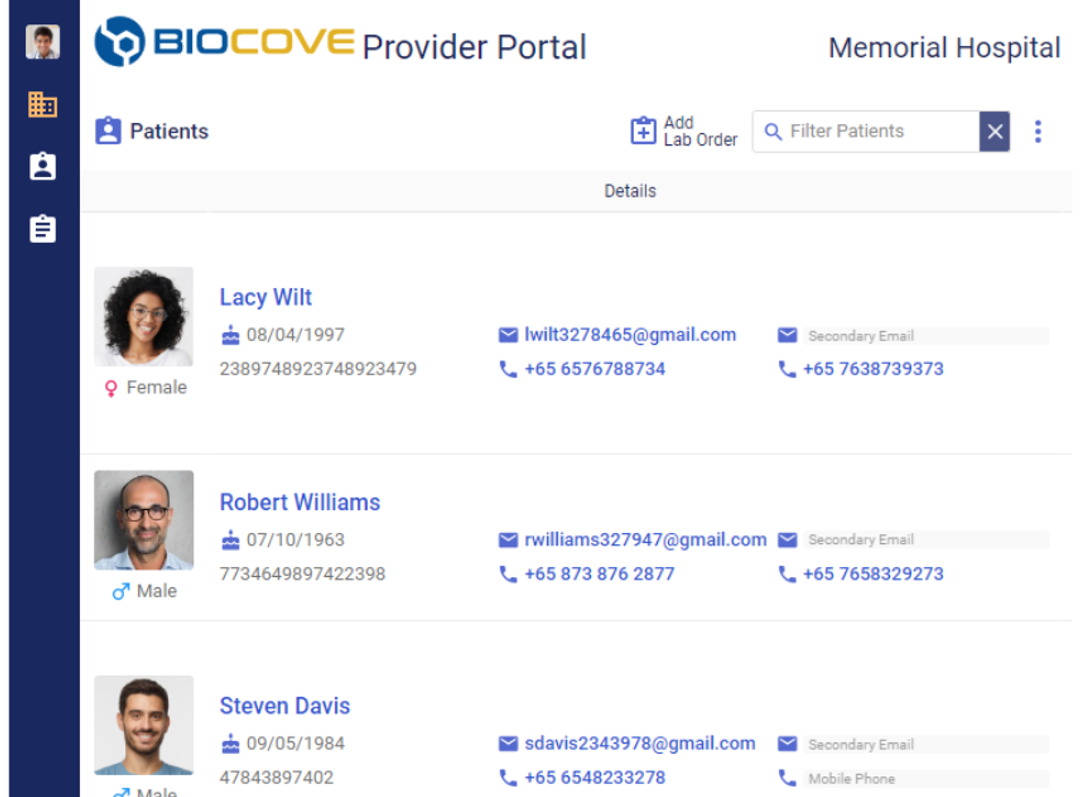
Physician Portal
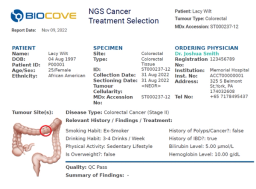
The Physician Portal unifies your clinical workflow from order placement and status tracking to report delivery. The Portal becomes a one-stop shop for your physician network, allowing them to create and submit lab orders and track test statuses and results.
Automated Accessioning
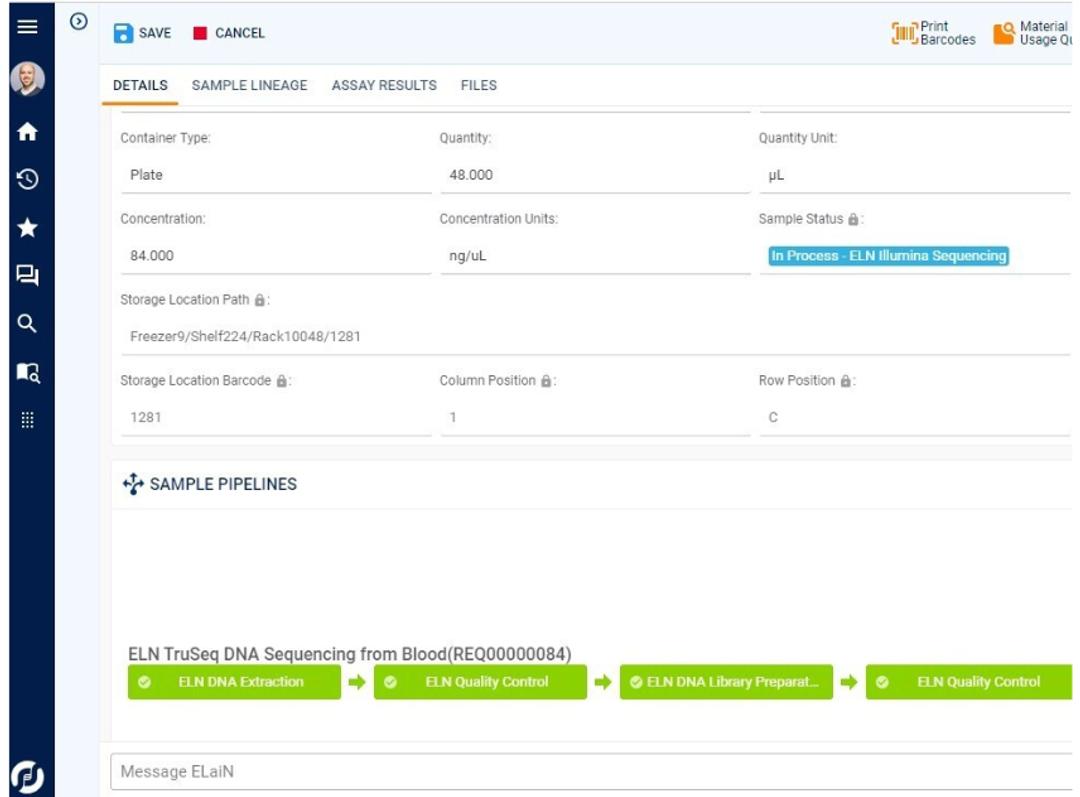
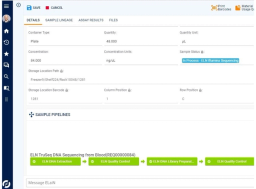
Electronic orders seamlessly synchronize with Sapio LIMS, where samples are accessioned, undergo order transcription, and flow directly into lab processing. Sapio automates the sample registration process, including identification, data capture and handling instructions. Samples are then accessioned automatically, including number assignment, validation and QC checks, and labeling. Sapio’s ELN manages instrument data processing, formulas, plating, and note-taking. After processing, results are analyzed and report documentation generated, which are reviewed and signed off before being returned to the Physician Portal.
Real-Time Sample Tracking
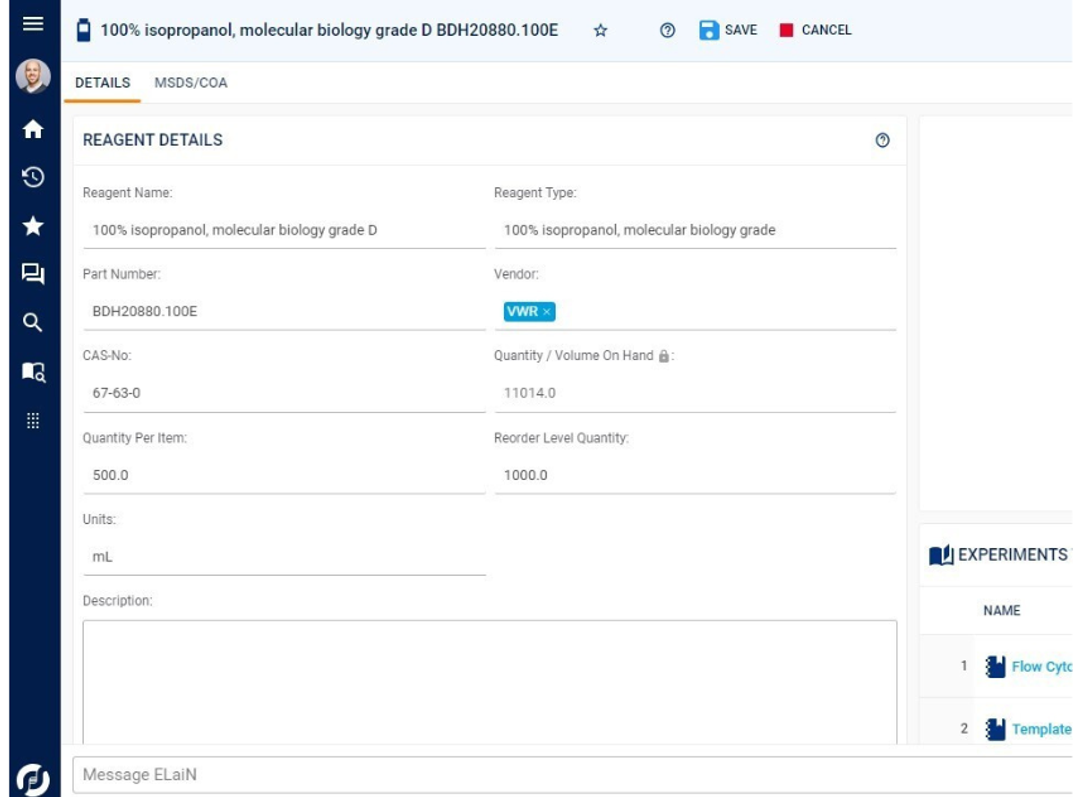
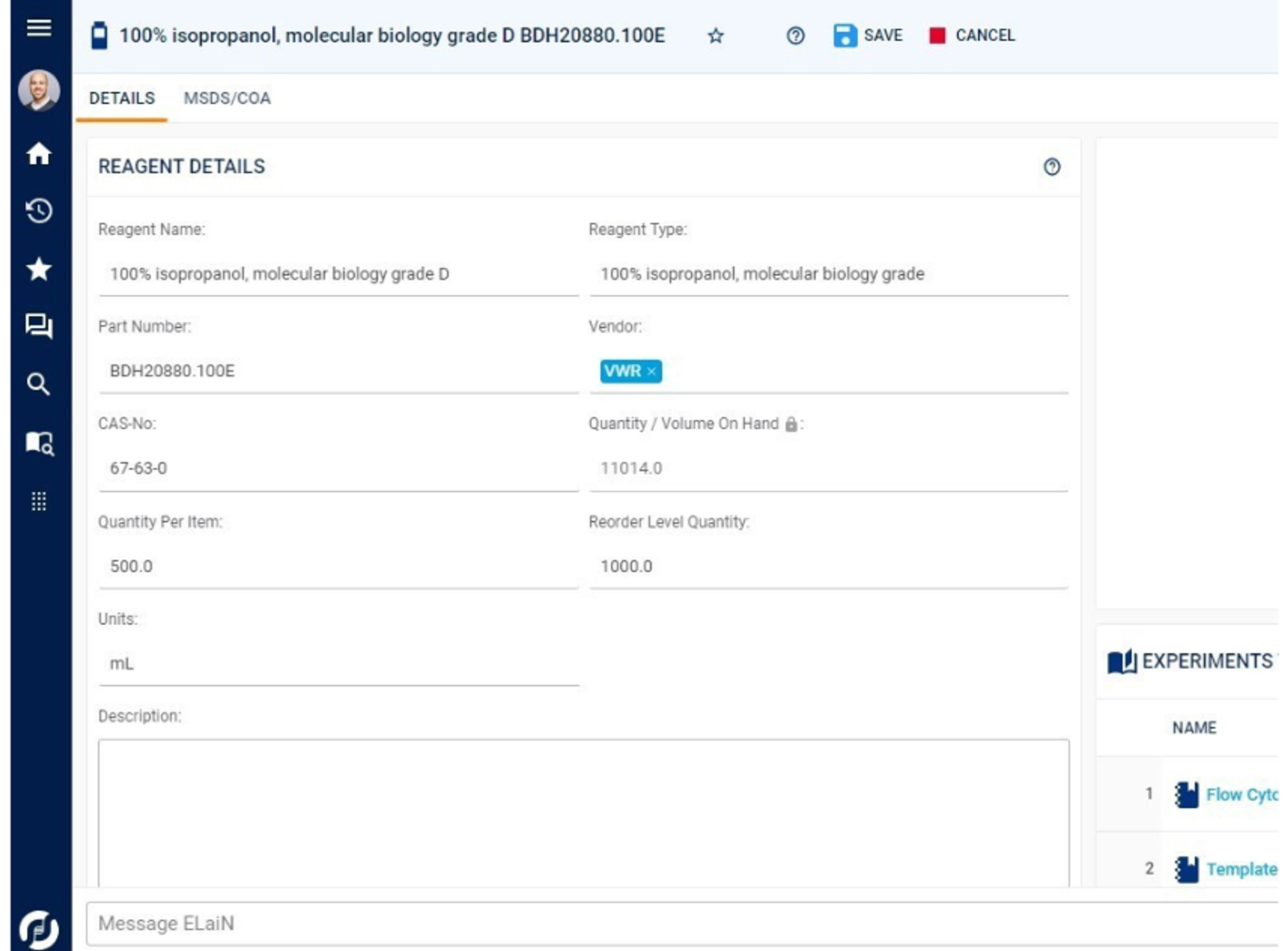
The management and tracking of samples, materials, and workflows end-to-end is core to the Sapio LIMS —from test order to final reporting. This includes chain-of-custody tracking and full sample lineage. Sapio delivers detailed information management and traceability across a full range of scientific data types and in real-time, including samples, reagents, containers, documents, and more.
GMP Compliant
Sapio LIMS is 21 CFR Part 11 and EU Annex 11-compliant, empowering labs to maximize quality and maintain compliance efficiently while maintaining their rapid speed of operations. With powerful GMP capabilities such as Quality Control (QC), Environmental Monitoring (EM) programs, and Stability Management, Sapio LIMS allows labs to uphold the highest standards for quality without disrupting scientists’ progress.
CRM Marketing Automation
Sapio expands end-to-end operations with clinic management, sales funnel management, and contact outreach with built-in digital marketing tools, including outbound and drip email campaigns, using an email template designer. A/B testing enables sales and marketing staff to set up different versions of your platform, collect data, and make better-informed decisions.
Comprehensive and Automated
From samples and materials, workflows, and plates to storage and instruments, Sapio LIMS easily automates and tracks your laboratory needs. Graphical storage management helps you find your samples or materials when needed. Instrument maintenance ensures your equipment is ready for your experiments.
No-code flexibility
Sapio LIMS features a drag-and-drop workflow builder, English sentence rules engine, configurable user interface, and natural language AI-chat (ELaiN), to simplify life in the lab. They aid scientists and lab operations in efficiently configuring processes and workflows, improving day-to-day operations, and enhancing data analysis and reporting, without the need for writing any SQL scripts.
Automated Reporting
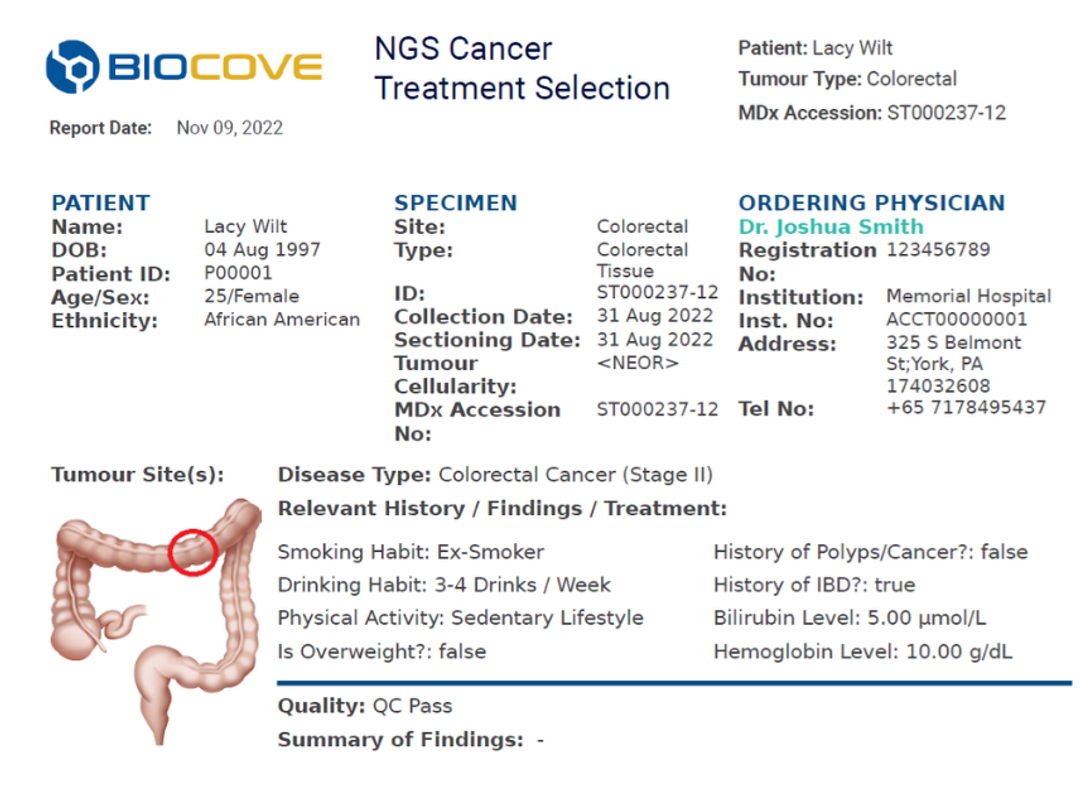
Automatically generate reports based on LIMS lab results. Data is integrated seamlessly across instruments and departments and is automatically validated and checked for errors. This data is then compiled into a clear and cohesive final report that medical directors can sign off. Signed reports are then automatically delivered back to the Physician Portal.