Products & ReviewClinical Diagnostics
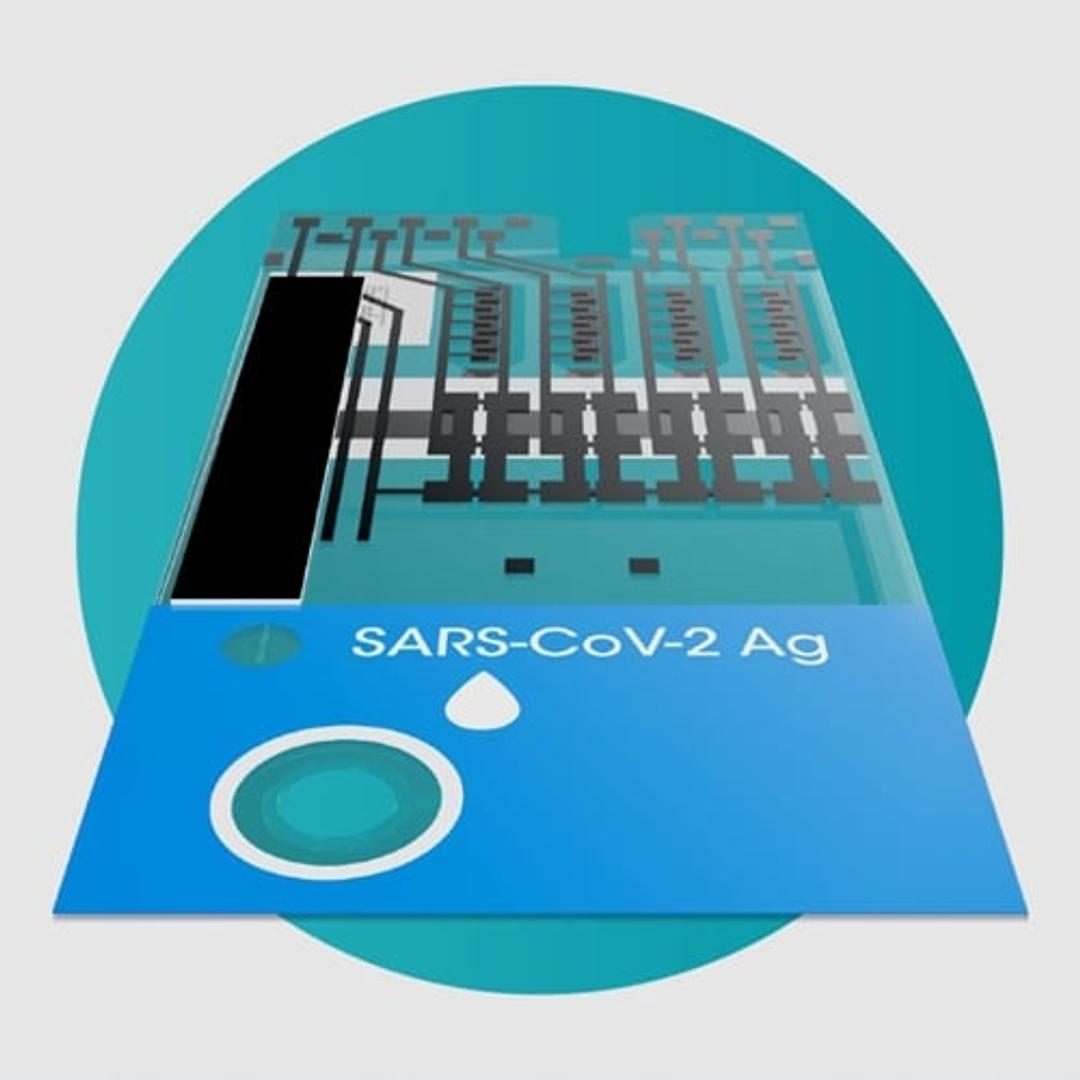
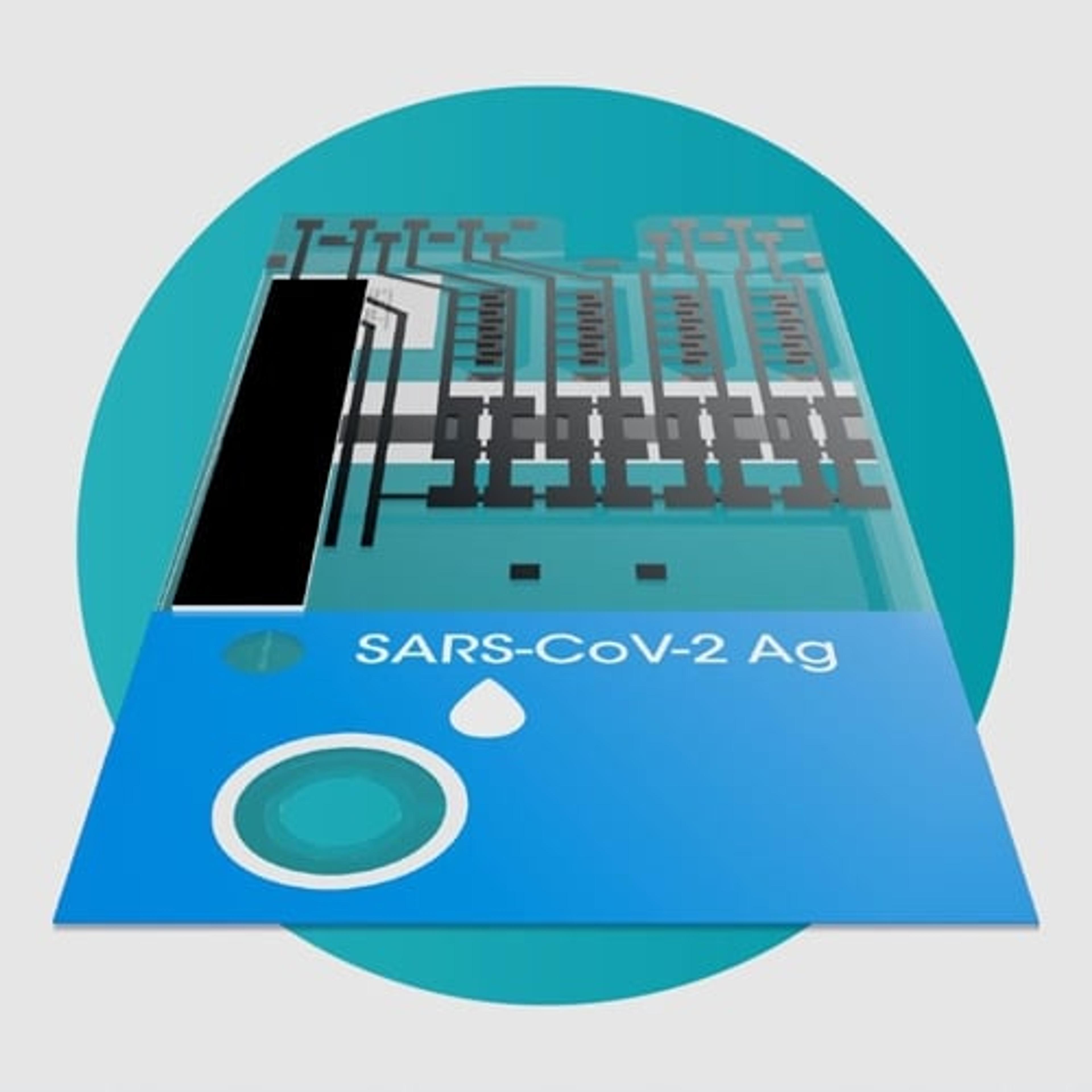
SARS-CoV-2 Ag Pool Test
The LumiraDx SARS-CoV-2 Ag Pool Test is a rapid microfluidic immunofluorescence assay for the qualitative detection of the nucleocapsid protein antigen in nasal or nasopharyngeal swab specimens pooled from up to 5 individuals suspected of COVID-19 or up to 5 asymptomatic individuals. Used with the LumiraDx Platform the test offers a rapid, scalable and cost-effective screening solution for infectious individuals.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
Test benefits
- Quickly and easily scale up testing: Low-cost diagnostic screening solutions
- Maximize resources: Pooled testing can improve access to testing by significantly reducing resources (tests, operators, instruments) required on a per patient basis
- Save Time: Pooled testing can result in a 40-60% reduction in testing, allowing for significant savings in both time and cost
- High sensitivity of up to Ct 33 enables the accurate detection of the majority of infectious patients
- Easy to implement in point of care settings
- Room Temperature test strip storage
- Time to result in 12 minutes
- Compact and portable instrument with connectivity options
- Facilitate reopening of work sites, sports, cultural venues and events and travel
- Regular testing of defined populations e.g. healthcare staff, healthcare facility residents, employees, students
- One time testing of groups for pre entry or reopening of sites