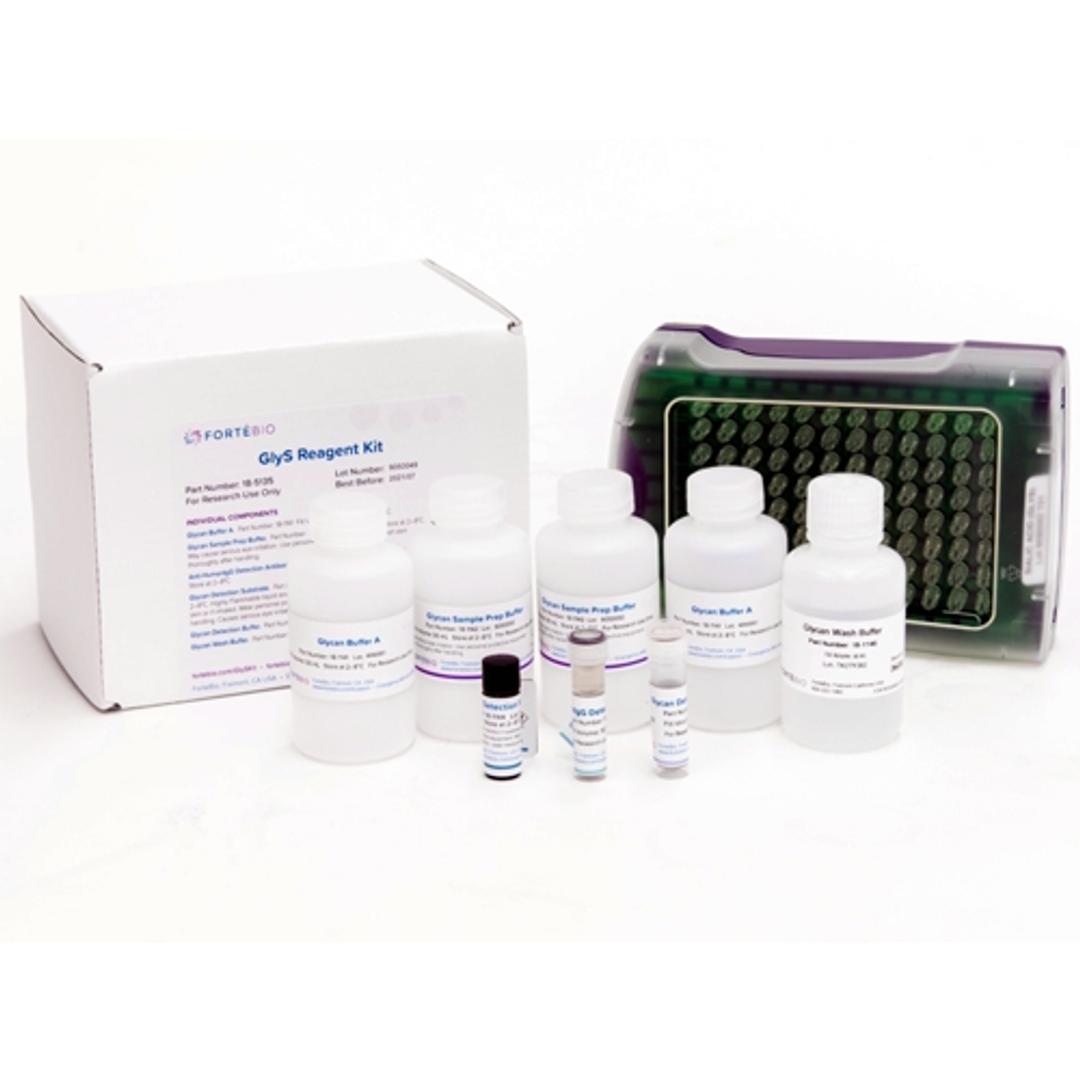
Sialic Acid (GlyS) Kit
High-throughput sialylation screening for expediting the clone selection process
Glycosylation is considered among the most important post-translational modifications when developing new biologics. Having a significant impact on product performance and variability, glycosylation is a critical quality attribute (CQA) influencing product safety and efficacy. Protein glycosylation can affect isolation and purification steps (process consistency), pharmacokinetics (half-life) properties and in vitro stability (product shelf-life). Sialic acid content is especially important as it can impact the stability and clearance of a protein.
ForteBio’s Sialic Acid (GlyS) Kit provides a rapid and convenient method for relative screening of terminal sialic acid content in crude or purified samples. This high-throughput screening method can readily be used to accelerate cell line development for producing therapeutic proteins with desired product qualities.
Benefits include:
- Work directly with cell culture samples: no need for sample purification/digestion steps
- Screen for samples with desired sialylation levels
- Combine titer data with sialic acid data for more informed decisions