Solentim iPSC Matrix
MatriClone and Clinical Manufacturing Grade Laminin* are xeno-free iPSC matrices comprised of the recombinant laminin-511 E8 fragment. The matrices are instrumental to the growth and maintenance of iPSCs and are suitable for single cell cloning of iPSCs, in addition to general iPSC culture. *Note, Clinical Manufacturing Grade Laminin can only be sold for use with Solentim Instrumentation.
MatriClone and Clinical Manufacturing Grade Laminin are manufactured following identical protocols, removing the need to re-optimise processes during the transition from process development to manufacturing.
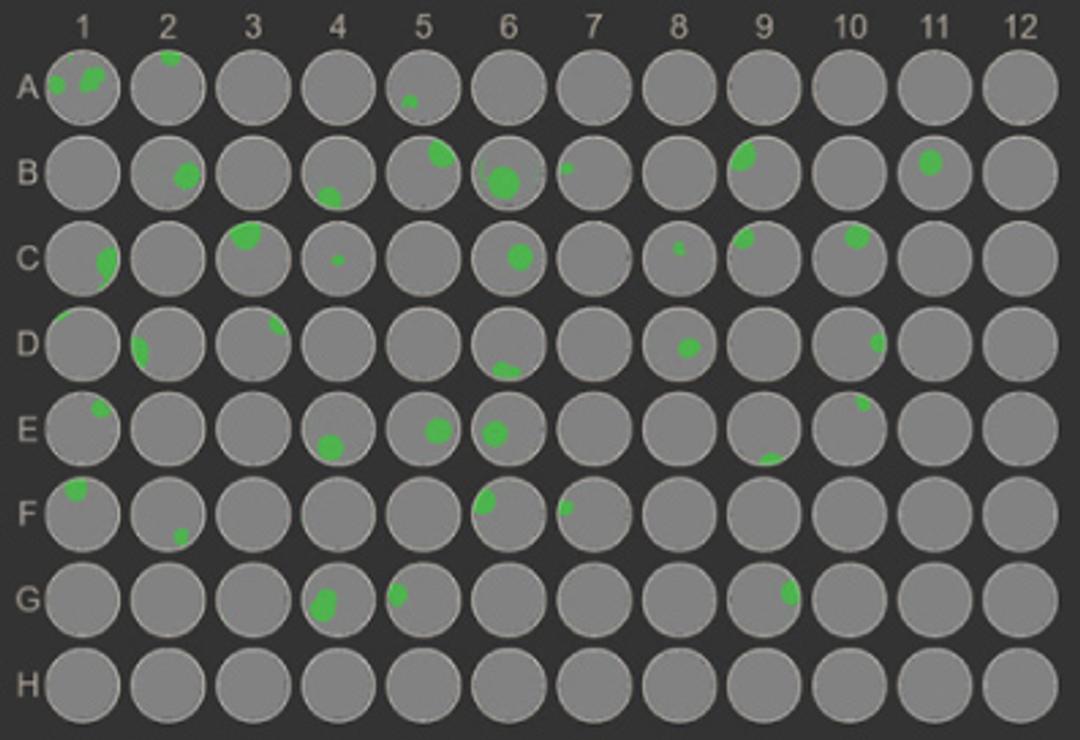
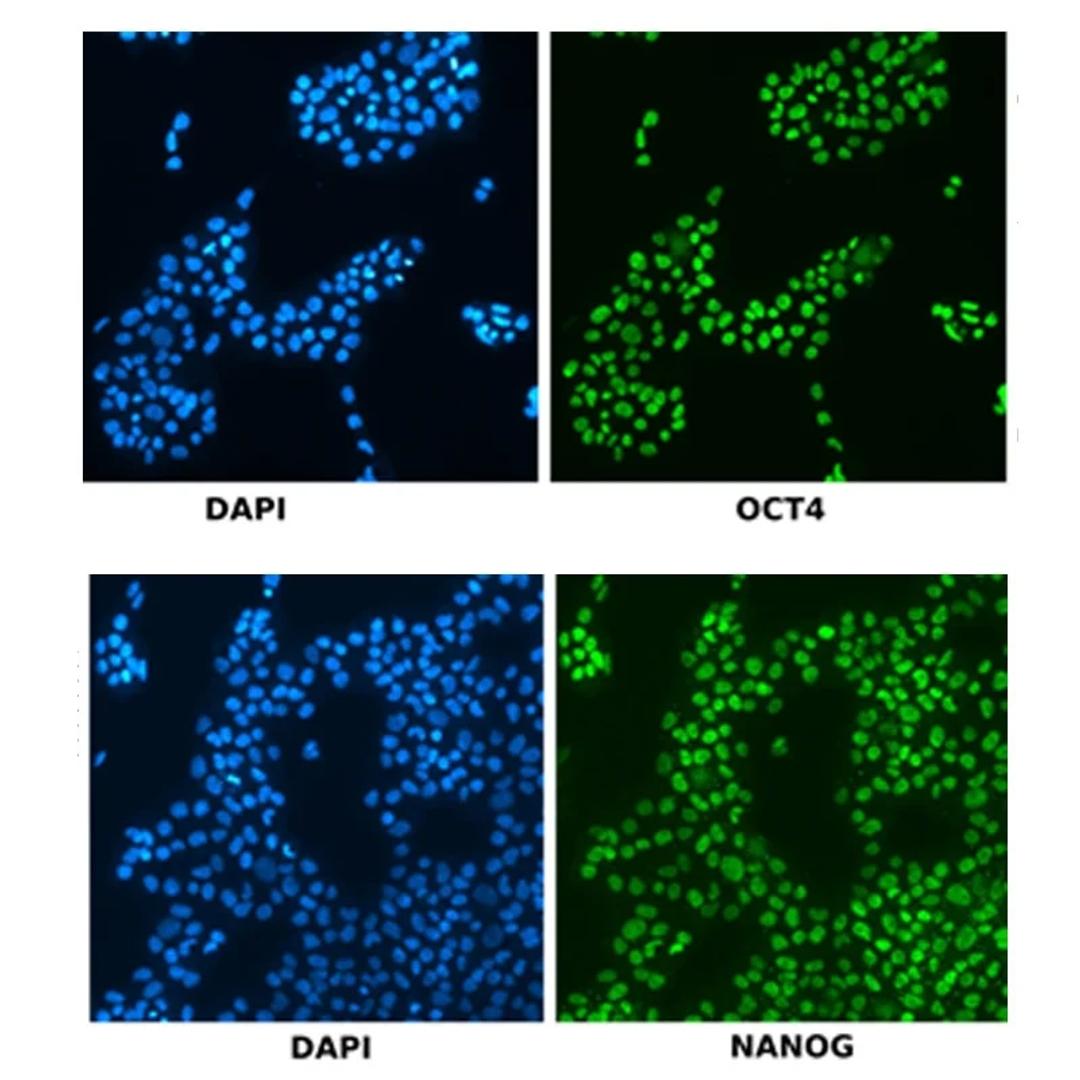
The matrices are optically suited for high resolution imaging and are therefore compatible with the VIPS® PRO and Cell Metric® X instruments for imaged based assurance of clonality.
Finally, the matrices are soluble, allowing the flexibility to be used either in-solution, or to pre-coat plates. Using MatriClone and Clinical Manufacturing Grade Laminin ‘in-solution’ not only saves time spent coating culture vessels, but also enables scientists to automate single cell seeding using the VIPS® PRO single cell seeder.
MatriClone
Product Details:
- RS-2100 – MatriClone 2 x 175µg
- RS-2101 – MatriClone 6 x 175µg
Product Application- Research
Product Specification
Sterility - Negative
Bacterial Endotoxin- No more than 5 EU/mg
Purity (SDS-PAGE) - More than 95%
Mycoplasma Test (PCR) - Negative
Integrin Binding Assay (Kd) - 1nM or less
Concentration- 500µg/mL (±10%)
Documentation
- Certificate of analysis (CoA)
- TSE/BSE
Clinical Manufacturing Grade Laminin
Product Details:
- RS-4100 – Clinical Manufacturing Grade Laminin 6 x 175 µg
Product Application - Manufacturing
Product Specification
Sterility - Negative
Bacterial Endotoxin- No more than 5 EU/mg
Purity (SDS-PAGE) - More than 95%
Mycoplasma Test (PCR) - Negative
Integrin Binding Assay (Kd) - 1nM or less
Concentration- 500µg/mL (±10%)
PLANOVA Integrity Testing- More than 1.40
Documentation
- Certificate of analysis (CoA)
- TSE/BSE
- Certificate of Standards for Biological Ingredients
- Certificate of Origin (COO)