STARLIMS Standalone SDMS
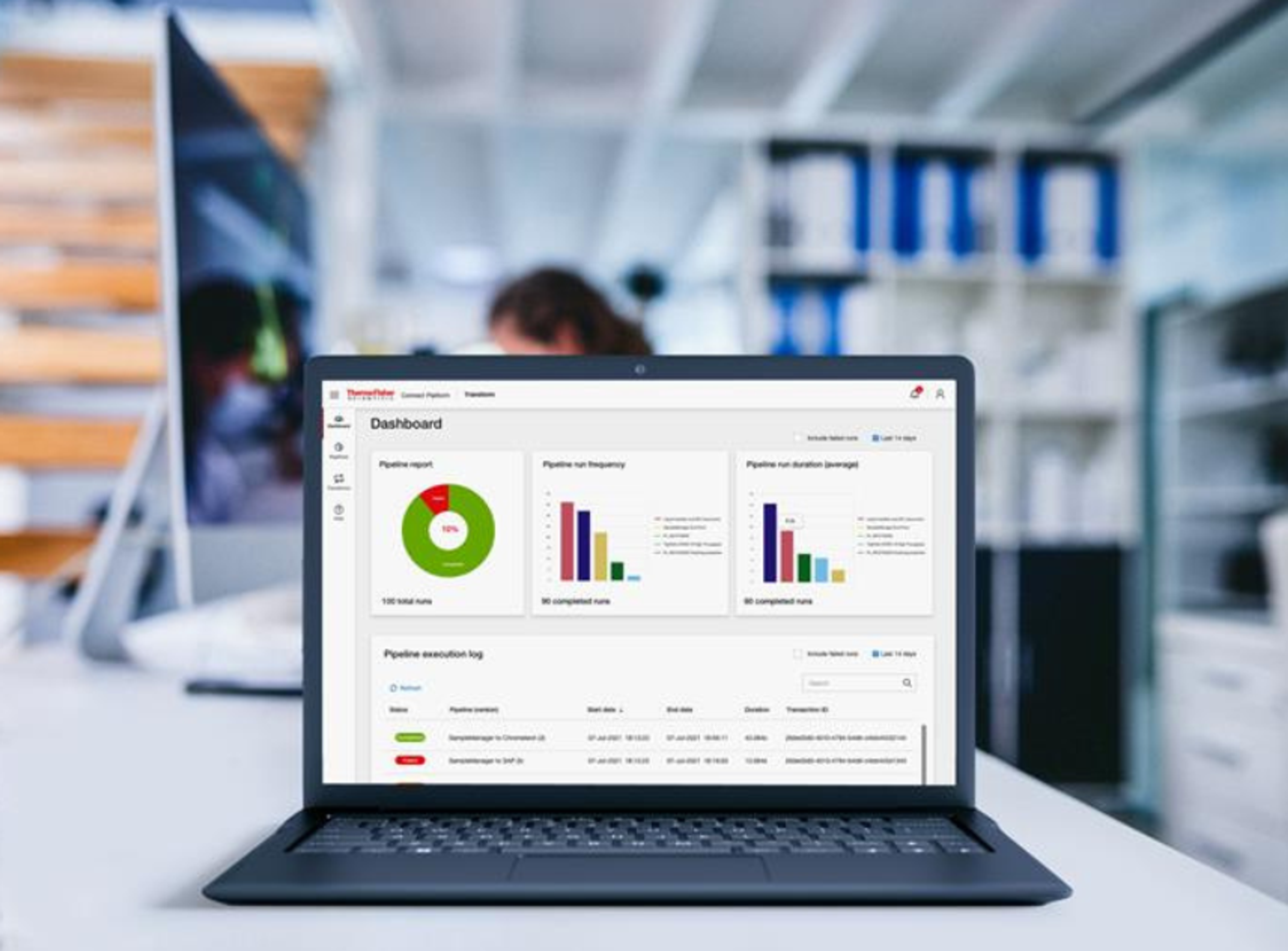
In addition to being available as part of STARLIMS Integrated Solution, STARLIMS SDMS (Scientific Data Management System) is now available as a standalone product to help clients achieve compliance with the data integrity expectations of the Food and Drug Administration (FDA) as well as other regulatory authorities.

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
In addition to being available as part of STARLIMS Integrated Solution, STARLIMS SDMS (Scientific Data Management System) is now available as a standalone product to help clients achieve compliance with the data integrity expectations of the Food and Drug Administration (FDA) as well as other regulatory authorities. STARLIMS SDMS V12.2 can work with an existing LIMS or without a LIMS, interfacing effectively with any LIMS (Laboratory Information Management System), CDS (Chromatography Data System), ELN (Electronic Laboratory Notebook), SAP (Systems Applications Products) and other lab systems through webservices without replacing them.
Key benefits of STARLIMS Standalone SDMS:
- It can identify and store equipment data files in native formats, parse data, include extracted metadata and does not require availability of the original software to create the record
- Enforces the document workflow and review process before the data is stored or pushed to native LIMS or ELN applications for an extra level of analytical quality testing
- Supports organizations to save on investing in additional middleware or multiple data storage systems
- Keeps your LIMS lighter and faster without replacing it
STARLIMS SDMS V12.2 can benefit three types of clients:
- Organizations purchasing the Integrated STARLIMS Laboratory Information Management Solution which includes SDMS.
- Organizations with an existing LIMS lacking SDMS capabilities.
- And organizations without a LIMS, seeking a Standalone SDMS solution to manage their data and better meet regulatory requirements.