Products & ReviewLife Sciences
Yale 2019-nCoV SalivaDirect ValuPanel Reagents
LGC Biosearch TechnologiesAvailable: Worldwide
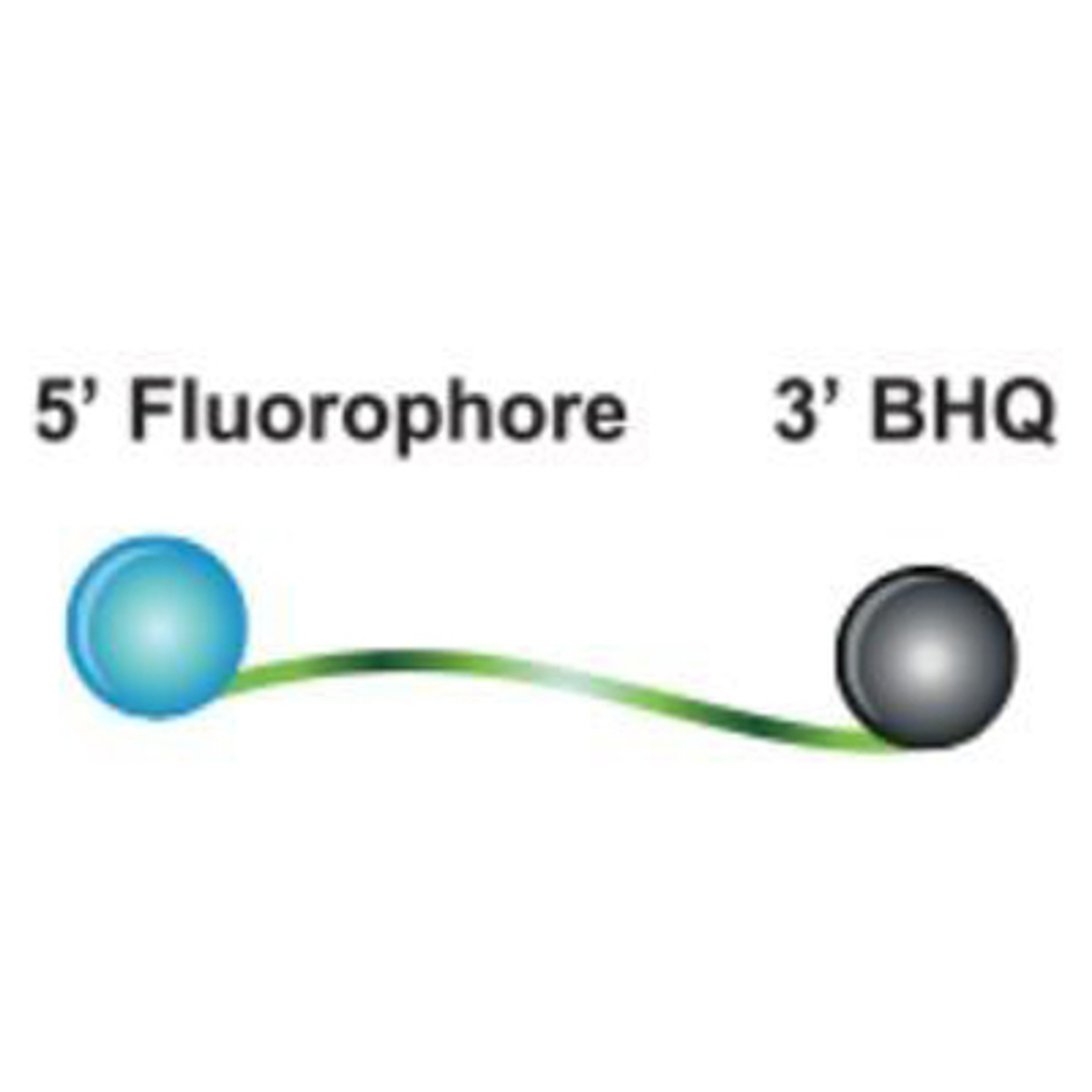
The Yale 2019-nCoV SalivaDirect™ ValuPanel Reagents™ include FAM-BHQ and Quasar™ 670-BHQ probes and primers delivered in individual tubes in dried format.
The oligonucleotide sequences were designed by US Centers for Disease Control and Prevention for use in real-time RT-PCR to detect SARS-CoV-2 viral strains.
The panel contains probes and primers comprising the following genetic signatures:
- SARS-CoV-2 N1 Primers (Forward and Reverse)
- SARS-CoV-2 N1 Probe
- Human RNase P Primers (Forward and Reverse)
- Human RNase P Probe (Quasar 670, SalivaDirect specific)
- SalivaDirect is a protocol, developed by the Yale School of Public Health, for testing people suspected of SARS-CoV-2 infection. While the method still relies on RT-qPCR, it uses saliva samples instead of nasopharyngeal swabs. SalivaDirect was issued an Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) on August 15, 2020.
Each reaction requires the following:
- N1 forward and reverse primers: 400 nM/reaction
- N1 probe: 200 nM/reaction
- RNase P forward and reverse primers: 150 nM/reaction
- RNase P probe: 200 nM/reaction